Fats
Dietary fats are contained in meats, eggs, nuts, seeds, dairy and fish and found in both solid and liquid (oil) form. They are a complicated topic owing to a great deal of cultural stigma surrounding them, and what we will attempt to do here is to give you an impartial overview of what dietary fats are, what they do and how they can affect us. Again, we will go into a little detail about the structure of fats and their digestive fate, but we will do this as simply as possible and hope we make a great deal of sense while making the topic interesting.
Historically, dietary fats have been blamed for increases in obesity and ill health, but we hope that the previous module has shown you that the crucial aspect of weight maintenance is not macronutrients specifically but the calories they contain. In fact, the link between fat intake and fat gain is literally down to the fact that, as you saw in the introduction to this module, dietary fat has twice the calories per gram compared to carbohydrates and proteins, and therefore they are easy to over eat.
Hardly a convincing case against fats…
Rather than being something which should be cut as low as possible in an attempt to reduce your chances of ill health, fats are a nutrient which should be considered vital (indeed, some are essential in the same sense as the above amino acids) but we must again be wary of being too hasty and we should remember that just because eating fat doesn’t cause fat gain directly, it doesn’t mean we get a free ticket to eat as much dietary fat as we want.
Fats should be considered just as important as the other macronutrients, and after explaining their function and role we will give you some recommendations for determining just how much you should eat and in what forms.
What is a fat?
Fats are an example of lipids (along with waxes and sterols) which are all organic molecules insoluble in water but soluble in organic solvents (in chemistry, ‘organic’ strictly denotes a molecule with a carbon base, it has nothing to do with whether something is alive, or whether something has been farmed in certain ways). The reason for this insolubility will be explained momentarily, but you may already be aware of it. If you have ever seen car oil sitting on top of a puddle, or have ever seen an oil/vinegar salad dressing separate where the oil sits on top, then you have seen the hydrophobic (water hating) nature of fats at work.
What this insolubility gives fats in our body is a very unique niche. Fats are used in specific orientations to create the membranes which surround every cell in your body, for example. Because of the hydrophobic property of the membrane, the contents of a cell represent a different environment to the rest of the body, meaning that special chemical reactions can occur there. Make no mistake, the ability that fats have to separate inside a cell from outside is literally the fundamental factor by which every single life form is able to function. From an evolutionary standpoint, the formation of small groups of fatty acids into a membrane is more than likely what led to the formation of the first living cell, from which all life eventually descended.
The fats which we consume in our diet are known as triglycerides which is a term you may have heard before, or the perhaps more accurate name tri-acyl-glycerol. The final term is more accurate because dietary fats are comprised of glycerol (sometimes called glycerine) attached to 3 fatty acids. Much like proteins, triglycerides are made up of hydrogen, carbon and oxygen, but they lack the nitrogen present in the former.
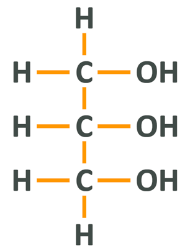
What you are looking at here is a 3 carbon chain. Attached to the first and third carbons are 2 respective hydrogens and the central carbon has only one because it is bonded to the carbons next to it (remember, each carbon is limited to 4 bonds). Moving to the right, you will see that each carbon group has a hydroxyl group, which is simply the name for oxygen and hydrogen bound together. To make this a triacylglycerol or triglyceride, each hydroxyl group becomes fused to a fatty acid
Structure of fatty acids and how we name them
Let’s look at the chemical structure of Oleic acid as a means of giving an example of fatty acid structure. Oleic acid is the fatty acid found in high amounts in olive oil, and is one of a few fatty acids which is associated with improved health.
Like all fatty acids, oleic acid has a certain structure. At one end, you have a carboxyl group, which is made up of a central carbon atom that has 1 oxygen double-bonded to it, and another oxygen which is single bonded to the central carbon and to an additional hydrogen atom (A C attached to an O with 2 lines, and also attached to an OH). After this group, there is a chain of carbon atoms which are attached to the maximum amount of hydrogen atoms possible according to their electron availability, and at the other end there is a methyl group which is a central carbon and 3 hydrogens. The amount of carbons in the chain varies between different fatty acids, but this carboxyl group – carbon chain – methyl group structure is how all fatty acids look. Sometimes the methyl group is referred to as the omega-end, which will become important momentarily.
Because each carbon has all of its outer ring electrons occupied the whole structure is extremely stable and as such will not dissolve in water, which would require some amount of bonding between the fatty acid and the free electrons on water molecules. It is this non-solubility in water that means fats can carry out the biological functions that they do and is probably the single most important fact about fats (it’s also why they are referred to as ‘fatty’).
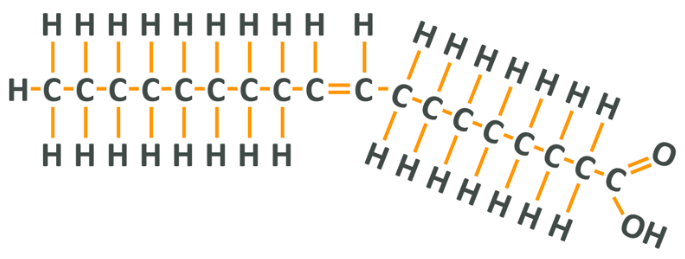
As you can see, oleic acid has its carboxyl group (COOH) on the right followed by a chain of carbons in single bonds. In the middle of the chain, however, there is a double bond which means that, because each carbon effectively ‘loses’ 1 more electron than it usually would, each can only bond to 1 hydrogen. The 2 lone hydrogens appear on the same side of the chain and repel each other, thus creating a ‘kink’ in the shape of the final molecule. After this, there is another set of carbons and a final carbon which forms part of the methyl group. Note that if you count from the methyl or omega end of the fatty acid, the double bond is on the 9th carbon.
When we know the chemical makeup of fats, we can group them into their respective categories:
- Short-chain fatty acids (SCFA) are fatty acids with a tail of fewer than 6 carbons (i.e. butyric acid)
- Medium-chain fatty acids (MCFA) are fatty acids with tails of 6-12 carbons
- Long-chain fatty acids (LCFA) are fatty acids with tails 13-21 carbons long
- Very long-chain fatty acids (VLCFA) are fatty acids longer than 22 carbons
If a fatty acid has only single bonded carbons it is saturated with the maximum possible amount of hydrogen and is therefore a saturated fat. If it has 1 double bond it is monounsaturated and if it has multiple double bonds it is polyunsaturated. If the double bond in an unsaturated fat falls on the 3rd, 6th or 9th carbon away from the omega end, it is an Omega-3, 6 or 9 respectively.
Oleic acid has a total of 18 carbons, which makes it a long-chain fatty acid. Because it has a double bond and therefore fewer than the maximal amount of hydrogens it is an unsaturated fatty acid (it isn’t saturated with hydrogen) and because it only has 1 double bond, it is a monounsaturated fatty acid. Because the double-bond occurs starting at the 9th carbon in the chain, it is a long-chain omega-9 monounsaturated fatty acid.
The amount of saturation and length affects the stability and structure of the fatty acid when exposed to cooking temperatures or the way they behave when used in the body. Generally speaking, saturation provides stability.
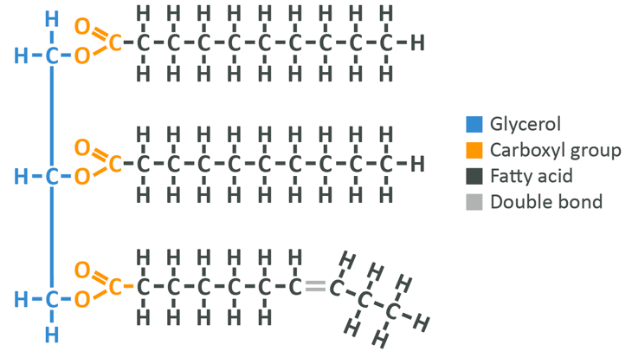
As you can see here, the glycerol remains on the left-hand side, but now the OH group has lost a hydrogen and the fatty acid has lost an OH group. This means that in total we have lost 2 H’s and an O. As you learned in the section on amino acids, this means that a water molecule has been created (not pictured), while the fatty acid has bonded to the glycerol.
Note: Each fatty acid bound to a given glycerol can be different. There is no need for all 3 to be the same molecule.
Now you have a broad idea of what a saturated and unsaturated fatty acid is, let’s look a tiny bit closer and introduce a new form of fatty acids – trans fatty acids.
What is a trans fat?
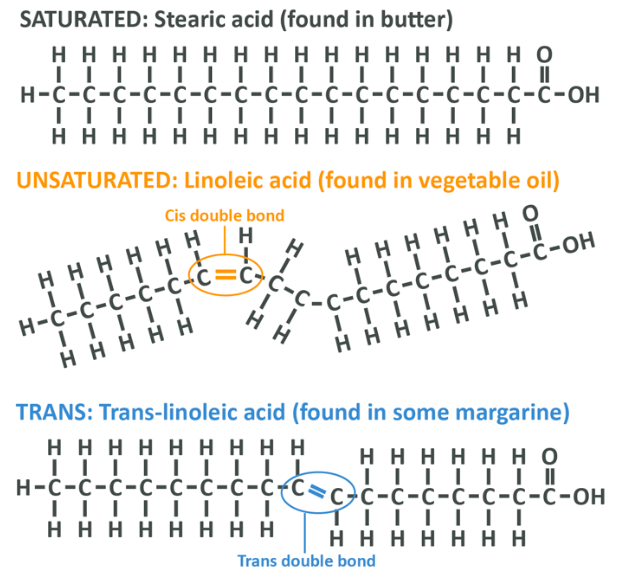
As you’ve already discovered, saturated fatty acids have no double bonds and therefore they tend to form straight chains, and because of this shape they are easy to stack on top of each other in an orderly fashion. This simple act of physics is why saturated fats are usually solid at room temperature – animal fat and coconut fat are made up largely (though not entirely) of saturated fat.
If a fatty acid is unsaturated, it means that there is a double bond which tends to create a kink in the chain, resulting in a liquid makeup at room temperature. In the simplest terms, the kink in the chain makes the acids much harder to stack together into a solid shape. This is how things occur in nature, but nature can be altered.
A ‘cis’ unsaturated fatty acid is one where the missing hydrogens in an unsaturated fat occur on the same side. Because they occur on the same side this leaves a gap, and the corresponding hydrogens on the other side of the chain are allowed to repel each other, causing a bend in the fatty acid chain and a liquid nature of the resultant oil as per the above – this is the standard way for things to be. In the food industry, fats which are solid at room temperature are preferable because they have a longer shelf life and better mouthfeel, but plant oils which are liquid such as sunflower oil or soybean oil are far cheaper to manufacture.
What food scientists discovered is that if you heat fats to a certain temperature in the presence of hydrogen, you can effectively break some of the double bonds and ‘saturate’ the fat with new hydrogens. This saturation removes the gap in the chain and straightens out the molecule, and as such, fully hydrogenated oils are solid at room temperature. This isn’t ideal, though, because these fats are more or less inedible due to being too solid.
What we needed was partial hydrogenation, hydrogenation to the point that a fat was hard enough but still edible.
When an oil is partially hydrogenated, we turn cis fats into trans fats. What this means is that rather than the missing hydrogens occurring on the same side of the chain, they occur on opposite sites. This still straightens out the chain and creates a solid fat, but it’s not so solid that you can’t spread it – success! Well, that is success in the eyes of food manufacturers. The impact these fatty acids can have on your health is another story. We will come to that shortly.
Notice in the image below that the 3 fats have the same number of carbons but their shape is different. In stearic acid the carbons are all fully hydrogen saturated, in linoleic acid there is a kink with 2 missing hydrogens on one side and in trans linoleic acid the hydrogens appear on opposite sides to each other, still resulting in a kink, but in a different direction and to a lesser degree. This tiny detail is very important because it changes the impact that a fatty acid has on your health. After all, we didn’t evolve to handle these molecules.
Before we move on, lets’ talk about one final molecule which you have no doubt heard of – cholesterol.
Cholesterol
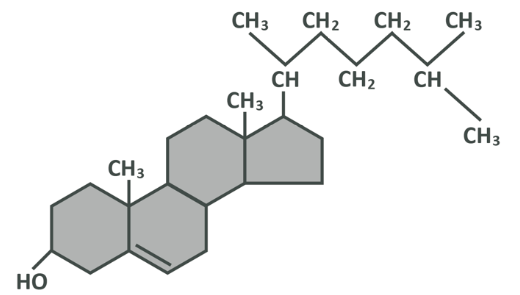
Cholesterol is, as the name suggests, a sterol (which is a lipid, just like fatty acids). Remember that a lipid is a compound of hydrogen, carbon and oxygen which does not mix in water.
We will not go into great depth about the structure of cholesterol, as all you really need to know is that it is made of three regions which you will see below – a hydroxyl group at one end which is soluble in water, 4 hydrocarbon rings (rings with carbon at each corner, single bonded to the carbons next to it and also bonded to 2 hydrogens which shoot off of it), and a hydrocarbon tail which is similar to the structure of a fatty acid. As you should now know, this chain is not soluble in water. Though cholesterol has parts which are and parts which are not soluble, for practical purposes here it is best considered to be a net insoluble molecule which will not mix into water (or blood for that matter).
The vast majority of cholesterol you have in your system is produced endogenously, that is you make it yourself. It’s produced primarily in the liver at a rate of about 1g per day, and you hold approximately 35g in your body at any one time. It’s used for vital functions such as making up part of the membrane of every cell in your body, transferring information from cell to cell and helping to create hormones. One of the other roles cholesterol plays is as a precursor for the bile salts released from the liver and gall bladder to digest fat.
It is worth noting that while most of the bile salts are re-used after the triglycerides being digested are absorbed, some are excreted meaning that some cholesterol is lost – in fact, this is the way the body ‘gets rid’ of any cholesterol it doesn’t need.
It helps us make cells, digest fat and produce hormones. It’s also possible to regulate the amount of cholesterol your body has within it by reducing endogenous production and increasing secretion if needs be.
Fat digestion and absorption
Now you know what a saturated, unsaturated and trans fat is and have an understanding of cholesterol, let’s zoom out a little and talk about how these are absorbed into your body.
Once you consume the steak we mentioned above, the fat within it (the triglycerides) make their way to the stomach and then to the small intestine – proper digestion of fats does not begin until this step. Once the triglycerides are in the small intestine they need to be absorbed, but because of the hydrophobic nature of these molecules, this is not easy to do. The bloodstream and the majority of your body is water based and so it is very difficult for triglycerides to enter them and they will generally ‘clump’ together as droplets of fat – in the same way that the ‘lava’ in a lava lamp forms blobs rather than mixing evenly.
This is why bile salts from the gall bladder are introduced along with enzymes called lipases, and the same neutralising bicarbonate ions mentioned earlier, to prevent your stomach acid destroying your intestinal walls.
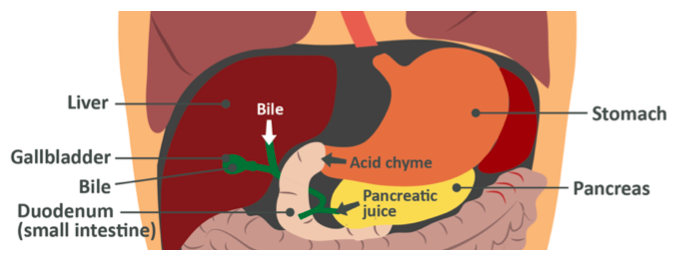
Note: The gall bladder does not actually ‘make’ bile, rather that is synthesized in the liver. The gall bladder acts as a reservoir for larger amounts of bile to be stored for quick-release via the common bile duct that you can see in the image above, linking both the liver and the gall bladder to the duodenum. If an individual has no gall bladder they still produce bile, but they are unable to maintain this stored ‘pool’ and as such, their fat digestive processes may be inhibited in terms of having large amounts of dietary fat per meal. This is not to say they can’t digest fat at all, rather, fat should be kept lower on a per-meal basis and eaten evenly though the day. This is a special case, though, and beyond the scope of this course.
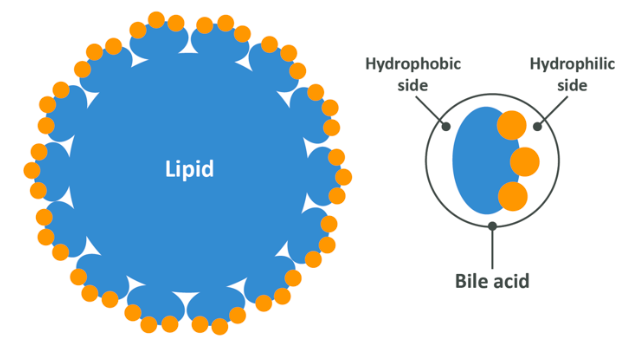
When bile is released into the small intestine, it acts as an emulsifier which means that it makes fats hydrophyllic (water loving). Soap is another example of an emulsifier, which is why applying soap to grease on a frying pan allows water to wash that grease away whereas it may have previously resisted rinsing. Bile does this very simply by virtue of the hydrophobic (water hating) side of a bile acid becoming attached to the hydrophobic surface of the triglyceride, while leaving it’s hydrophyllic side exposed. This compound of bile and triglyceride is now able to be mixed into water easily. In the image below the bile salts have completely encased the lipid and only the hydrophyllic side is exposed.
Now that this triglyceride is hydrophyllic, the lipase enzymes are able to get to work breaking the bonds between the fatty acids and the glycerol backbone, leaving two free fatty acids and one which is still bound to the glycerol, now known as a monoglyceride. These are now molecules which are small enough to be absorbed into the cells embedded in the intestinal wall. Any dietary cholesterol will be absorbed at this point, too, along with the ‘fat-soluble vitamins’ A,D,E and K. We will talk about these a little more in the module on micronutrients, but for now it’s clear that without dietary fat you cannot absorb these very effectively, meaning that you are at risk of deficiency even if you are eating foods which contain a reasonable amount of them.
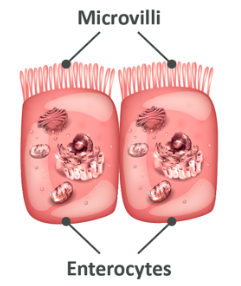
In the image above, you are again looking at cells of the intestinal wall, the enterocytes.
As you already learned, the ‘wavy’ part at the top are known as villi and these serve to increase the surface area to allow for easier absorption. Once the free fatty acids and monoglycerides are absorbed into the cells of the intestinal wall, the next step is to absorb them into the blood.
The fatty acids and monoglyceride enter a cell structure (present in most cells, not just these ones) called the endoplasmic reticulum, which then re-packages them into triglycerides by linking them back together and sends them on to another structure called the golgi apparatus. The golgi apparatus combines them with cholesterol and coats them in a protein to make them water-soluble. This final form is known as a chylomicron which is a lipoprotein (a term we’ll return to later), and these water-soluble chylomicrons are then fed into the lymphatic system (a huge system of tubes carrying lymphatic fluid or lymph all around your body) which leads to the blood and finally to storage sites, such as muscles, your liver or most often, your bodyfat or adipose tissue.
Once the chylomicron (a protein-encased bundle of triglycerides and cholesterol) reaches the site in which it will be stored or used, enzymes called lipoprotein lipases break down the triglyceride once again, allowing them to be absorbed into the cell and used, or recombined a final time and stored. The chylomicron could now be considered ‘empty’, but this is not quite the case, as there will still be some remnants of triglycerides and cholesterol present which are useful to the body. This chylomicron remnant is delivered to the liver, and we can ignore it just for now.
Dietary fats are therefore eaten, emulsified by bile, broken down by enzymes into free fatty acids and monoglycerides, absorbed into intestinal cells, rebuilt into triglycerides, packaged as chylomicrons along with cholesterol, sent to lymph, sent to blood and finally transported around the body to be delivered to cells which will either use them for energy or store them. This storage is important, because although we can use fats for energy immediately after we eat them, we often go numerous hours between meals and for that we need to talk not only about the way our body handles fat in the ‘fed state’ after a meal, but also in the fasted state between meals.
Each gram of stored triglycerides is potentially worth 9kcal, and because fats are hydrophobic, they can be stored with no additional water (though in adipose tissue they are accompanied by some proteins and cellular structures). As such, 1kg of fat tissue will be around 87% fat and therefore yield somewhere around 7800kcal for use when needed. As you can probably imagine, it is through this process that the human race and those before it was able to survive famines, and between meals this is partly what allows us to fuel our cells despite a lack of immediately available food. This potential for being a vital energy source can be considered one of the main functions of dietary fat, alongside the use of fatty acids for forming cell walls, aiding with cellular communication and other similar roles.
Back on topic, fat which has been stored can be used as fuel but for that to happen, we need to release fat from our fat cells again…
Once fat has been stored, how do we use it?
Most of the fat you have in your body is stored in bodyfat tissue or adipose tissue. This is a dense network of fat cells known as adipocytes which are best considered to be like a bunch of balloons packed tightly next to each other, each containing some fat. The contents of each increases or decreases over time as you gain or lose fat. This should help you understand that your fat cells (in adulthood) typically get fuller and emptier as you gain and lose stored bodyfat, rather than it being the case that you create new cells if you gain additional fat. You may remember this principle from the last module where we mentioned that the reduction of fat in the fat cells throughout your body is one of the primary signallers for your body to start resisting fat loss.
Most of the fat stored in these cells is in the form of the triglycerides which we have just explained. Once they are packaged by the golgi apparatus and transported to the blood via the lymph, they are carried through the bloodstream to fat cells around your body and deposited (without the ‘casing’ that they were packaged with before ‘travel’).
Between meals, during exercise or a times of low overall energy intake (such as when you are eating in a deficit) your fat cells receive a signal to start releasing their stored energy. In order for your body to now use these fats, the first step is to remove the 3 fatty acids on a triglyceride molecule from the glycerol backbone – this is, as you can probably imagine, again performed by enzymes, in this case called lipases (you were introduced to these as the enzymes responsible for breaking down triglycerides in the intestine). You may recall that in order to link fatty acids to a glycerol it is necessary to waste a molecule of water (H2O) as 2 hydrogens and 1 carbon were lost; knowing this, your logic should enable you to work out how the lipases remove the fatty acids, they add water or hydrate them in a reaction known as a hydrolysis reaction.
Once the triglycerides have been hydrolysed, we are left with 3 fatty acids and a unit of glycerol which makes its way to the liver (we will discuss the fate of this glycerol in the next macronutrient section). The fatty acids are packaged into protein and delivered around the body for use as an energy source.
So far, we have explained that fat is eaten, absorbed into your intestines and then sent, via the lymph, around the body in special protein cases. It’s used by cells for energy, or stored in specialised cells to be used later. We have explained two ways which you might end up with fatty acids in the blood – either you have just eaten and are in the ‘fed state’ during which time fatty acids in the form of triglycerides packaged as chylomicrons are being transported in your blood for use or storage, or you may be in the ‘fasted state’ between meals at which point fatty acids are being released and transported via the blood in albumin for use as energy – but there is another way that we can have fat in our blood, and in order to explain that we need to talk more about your liver.
Lipoproteins
To explain this issue, we must explain a little more about lipoproteins. As you may be able to tell from the name, lipoproteins are made up of fat or lipids (lipo-) and proteins. Lipoproteins are fats (which you remember are insoluble due to being largely or entirely hydrophobic) encased in a protein so that they can be transported around your blood.
There are five key versions, one of which you have already met. These are chylomicrons (the protein-encased triglycerides which are made within the cells lining the intestinal wall during the initial process of fat digestion), Very Low Density Lipoproteins (VDL), Intermediate Density Lipoproteins (IDL), Low Density Lipoproteins (LDL) and High Density Lipoproteins (HDL). Some of these terms may be familiar to you as it is these lipids which are measured in your blood to determine disease risk.
The density of each can be considered to refer to the amount of protein contained in each, in comparison to fat. Additionally, the fat:protein ratio scales with the size of the molecules, with the relatively highest fat molecules being physically larger than the relatively highest protein molecules.
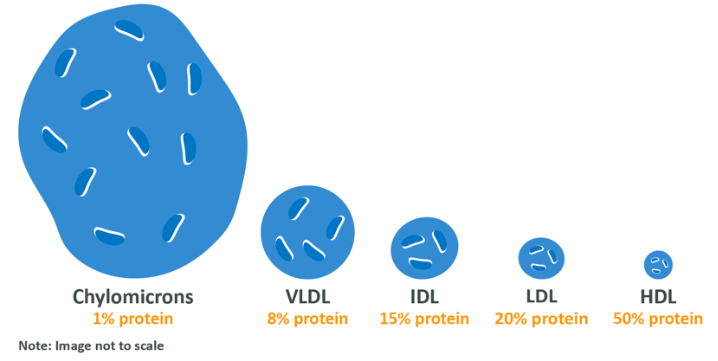
We’ve already discussed the life of a chylomicron but briefly, it is created in the cells of the intestinal wall by combining triglycerides, cholesterol which we have eaten and a few other lipids and encasing them in protein. This is then absorbed into the lymphatic system followed by entering the blood, and transported around the blood depositing lipids into various cells. Any chylomicrons which are not completely emptied then return to the liver where they are detected by special receptor sites and absorbed.
Within your liver cells a number of reactions take place which are dependent on a ready supply of carbohydrate and a number of enzymes. These reactions result in the production of cholesterol, fatty acids and monoglycerides. These monoglycerides and fatty acids can then in turn be combined to create new triglycerides which are then packaged with the newly created cholesterol (and some dietary cholesterol which came ‘on board’ a chylomicron) to produce 2 new lipoproteins – either VLDL or empty HDL molecules.
Remember that VLDL has a lot more triglycerides than does HDL, and this is because it’s role is to transport those triglycerides to your cells or tissues for use as an energy source in almost the exact same way as a chylomicron except rather than taking triglycerides which have been ingested very recently to cells, it takes triglycerides which have been synthesised in the liver either from old chylomicron remnants or from reactions within the liver itself. Of course, triglycerides not used for energy are subsequently stored.
As a VLDL travels around your blood, it will come across the same lipoprotein lipases which will break off some of the fatty acids stored within it and donate these to cells. When this happens, the VLDL loses some lipid density and becomes IDL which eventually becomes LDL. LDL’s job is to transport cholesterol to cells which need it in order to allow them to repair their membranes, build hormones and so on. After LDL donates its cholesterol, it returns to the liver to either be recycled or excreted (along with the cholesterol it still carries) through bile. You can conceptualise this as a VLDL leaving the liver ‘full’ and gradually ‘emptying’ before returning to the liver.
The HDL is considered to be a ‘good’ lipoprotein often simply referred to as ‘good cholesterol’. It’s role is to circulate in the blood and pick up excess cholesterol from cells to bring back to the liver for use or excretion. As such, it has the ability to lower your blood cholesterol levels.
As you can see, cholesterol per se isn’t necessarily the big problem it is promoted to be. It’s produced in the liver (and a small amount is eaten in the diet), the levels of cholesterol are pretty tightly controlled throughout your entire body through reduced production and an ability to excrete what isn’t needed, and it serves a lot of key functions, so why do people worry about cholesterol in their blood?
Atherosclerosis
Atherosclerosis (coronary heart disease) is the narrowing of the blood vessels surrounding the heart which occurs due to a build-up of plaque. It is one of the most common cause of heart attacks, can lead to strokes, and is one of the biggest killers in the western world.
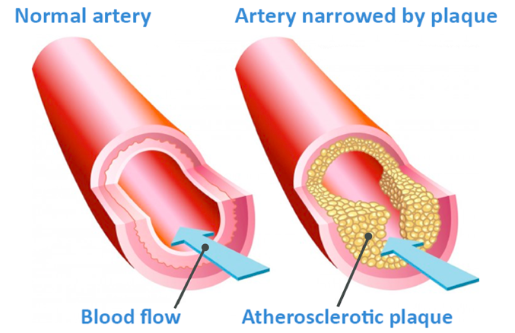
Atherosclerosis occurs when either abnormal lipid profiles, irritants such as smoking or conditions like hypertension damage or irritate the inner wall of a blood vessel, allowing LDL (but also other non-HDL lipoproteins including chylomicron remnants) to pass through the thin inner layer of the artery and start to become oxidised. Oxidised lipoproteins are toxic and therefore these initiate an inflammatory response, attracting white cells from your immune system.
The white cells ‘eat’ the lipoproteins present in the cell wall in an attempt to clear them out. Unfortunately, this is not as effective as we would like, and the white cells swell up (when collected they appear like foam under a microscope, hence the name ‘foam cells’). When these cells die, they spill their lipid content into the wall of the artery, creating what is known as a lipid core. A fibrous cap forms over this core, attempting to heal this lesion, but as build-up continues the fibrous cap is unable to prevent the plaque from protruding into the artery.
As you can see, atherosclerosis is a major problem, but as you can also see it’s not the molecule cholesterol which is causing the issue, but rather the lipoproteins which carry it. While an elevated cholesterol count was once thought to be a problem by itself, what we now understand is that the amount of cholesterol in your blood is only one potential risk factor, as the cholesterol in your blood may be in different places.
Recall that VLDL, IDL, LDL and chylomicron remnants (collectively known as non-HDL cholesterol, non-HDL lipoproteins or non-HDL-C) can carry cholesterol in your blood and deliver it to the body, while HDL is responsible for carrying cholesterol from your body back to your liver. Because of this, your non-HDL-C count and your HDL counts are likely to be a far more reliable measure of your heart disease risk than cholesterol alone. Total cholesterol measures do not account for HDL which is responsible for removing excess oxidised lipoproteins and cholesterol from the site of atherosclerotic plaque, and therefore they may not be reliable (which is absolutely not to say they should be discounted outright).
In fact, an abnormal lipid profile (elevated non-HDL and/or reduced HDL) is one of the biggest risk factors for atherosclerosis, and is so independent of changes in bodyweight, though this is not to say that bodyweight is not important.
Remember that VLDL and IDL as well as chylomicron remnants carry a significant amount of triglyceride, while LDL carries comparatively little, mostly carrying cholesterol? Well, high levels of blood triglycerides (hypertriglyceridemia) is also a major risk factor for atherosclerosis, and the primary cause of this is metabolic syndrome and diabetes, which results from over-nutrition, low physical activity and obesity. Because of this final factor, it’s now widely considered that the two key markers for potential atherosclerotic issues are blood triglycerides and serum HDL, while serum LDL is of course still a very relevant issue.
In short, to avoid atherosclerosis the key things are to maintain a healthy weight as we mentioned in module 1, and maintain a healthy lipid profile. But how do we do that?
Fatty acids in the diet and your blood lipids
Hypertriglyceridemia is caused largely by your diet. There are familial traits which can influence your blood lipid levels but that is far beyond the scope of this course. What matters is, now that we know a healthy level of triglycerides in the blood (transported by all lipoproteins) and a healthy level of both HDL and LDL are required to improve health, we can ask the question – how can we make sure we do this in the context of our lifestyle choices?
Very briefly, before we move on, smoking, a high consumption of alcohol, extreme levels of stress and simply being overweight and/or inactive can cause both an impairment in HDL/LDL balance and an increase in triglycerides, and as such leading a lifestyle which avoids stress and the overconsumption of drugs is important. Also key, is maintaining a high activity level. The WHO recommends at least 30 minutes of moderate activity daily, with the ideal being 30 minutes intense or 60 minutes of moderate exercise. Just as crucially, it’s important to maintain a healthy weight and avoid extended periods of time involving excessive food intake in general (while you do need to overeat if you choose to gain muscle, this is a moderate increase and not the topic of this discussion. Here we are talking chronic overeating resulting in excessive nutrient intake and therefore fat gain). Now back to the focus on diet.
It’s been long understood that aside from total calorie intake, one of the primary drivers for an impaired blood lipid profile is dietary fat intake.
Initially it was known that increased saturated or unsaturated fat caused an increase or decrease, respectively, in serum cholesterol levels (hence the abovementioned recommendations to cut out saturated fat and cholesterol). Later, when it became possible to separate lipoprotein fractions and study them, it was ascertained that LDL and VLDL were the primary corollaries with heart disease. Later, it was discovered that total cholesterol to HDL ratio (or triglyceride to HDL) was a better indicator of heart disease risk, meaning that it was possible to study the lifestyle and dietary factors which impacted upon these specific markers, and by the 1990’s the mechanisms by which dietary fatty acids were impacting upon lipid levels was starting to be understood.
When your body’s cells are starting to run low on cholesterol, a signal is sent from them which increases cholesterol synthesis in the liver. This signal can be regulated by consuming unsaturated fatty acids, and therefore these fatty acids can reduce serum cholesterol levels through their ability to reduce the rate at which cholesterol is synthesised in the liver. It’s recently been discovered that short-chain fatty acids (we will return to these when we talk about dietary fibre) and medium-chain saturated fats seem to have a similar effect to this, too. On top of this, it’s well established that a diet high in saturated fatty acids increases LDL.
This would seem to suggest that consuming low amount of saturated fat (or zero saturated fat) and higher amounts of unsaturated fat would be the most health-promoting approach, but this is perhaps hasty.
To begin with, this ignores the impact that dietary fats can have on HDL, instead focusing only on LDL. It also ignores the fact that all fatty acids within a group are not the same. For example, stearic acid and lauric acid may both be saturated fatty acids which increase total lipid levels in the blood, but stearic acid does not increase HDL whereas lauric acid does, significantly.
Finally, though risk factors and blood markers are crucial, they are not everything. We aren’t really concerned about some particles in our blood, we are concerned with the impact that fatty acids actually have on our long-term risk of getting diseases, and when we think in these terms the waters get a lot more muddied. Two high quality reviews of the data which looked at the association between saturated fat and heart disease found no correlation at all. Although saturated fat can increase serum lipid levels, it may not always impact heart health directly due to mechanisms which we simply don’t yet understand.
Here’s what we know about the effects different fatty acids may have:
- It’s known that polyunsaturated fatty acids can lower total blood lipids, but when they do this they lower HDL alongside LDL. Not only this, but it is the polyunsaturated fatty acids contained in LDL lipoproteins which can oxidise when the LDL enters the arterial walls, which is the reason that white cells arrive and the plaque build-up occurs – clearly, overconsuming polyunsaturated fatty acids isn’t a great thing, but at the same time they do indeed function to keep total blood lipid levels in check so they should be consumed in some amount, and certain polyunsaturated fats such as the Omega-3’s we’ll mention momentarily have beneficial properties all of their own
- Saturated fatty acids raise total blood lipid count, but they may also increase HDL depending on the specific acid. In some studies, high intakes of saturated fatty acids are associated with increased cardiovascular disease but when associating saturated fat with heart disease it’s very difficult to isolate saturated fat as the only factor – saturated fat consumption is also associated with processed food consumption, smoking and a sedentary lifestyle which all serve to make this data less cut and dry
- Saturated fatty acids are vital for cell function, cell wall production and cellular signalling. Additionally, saturated fatty acids transported in LDL are not able to oxidise, and therefore cannot directly lead to atherosclerosis themselves (though an increased LDL concentration leads to an increase in all fatty acids being present in the blood)
- All of this adds up to say that while the idea that saturated fats are not a direct cause of heart disease by themselves holds water and they should not be excluded from the diet completely, it cannot be ignored that they may be harmful when consumed in excess. The ‘ideal’ intake for saturated fat may not be high, but it also cannot be considered to be zero
- Monounsaturated Fatty Acids (MUFA) seem to have a lowering or at least neutral effect on total cholesterol. They also raise HDL while lowering LDL, meaning that they tend to be considered ‘the good guy’ in all of this. MUFA are present in high amounts in Mediterranean style diets which are themselves considered to be extremely healthy approaches to nutrition, and it’s a reasonable assumption that MUFA inclusion is at least one small factor in that
- Trans fatty acids will increase total cholesterol by increasing LDL while also decreasing HDL. Trans fatty acids produces in food manufacturing are generally considered to be a net negative, and their consumption could be considered a risk factor for heart disease by itself. Trans fatty acids, because of their unnatural shape, are very difficult for your body to deal with, and avoiding them as much as is reasonably possible (eating a doughnut once a month isn’t going to kill you) is an exceptionally wise idea
Other than trans fatty acids, as we hope you can see, the research into fatty acid consumption is hugely nuanced and difficult to really pull together. It’s generally considered that saturated fatty acids increase serum cholesterol levels which is a really strong predictor of heart disease, but at the same time the population data doesn’t support the saturated fat/heart health link. Polyunsaturated fatty acids lower total cholesterol, but they also lower HDL and are susceptible to oxidation. Monounsaturated fats and trans fats appear to be a little more cut and dry as beneficial and harmful respectively, but it’s still very early to draw strong conclusions about MUFA being a panacea of health due to limited strong long-term data.
What about Omega-3?
To throw yet another factor into the mix, let’s talk about Omega-3’s. Omega-3 fatty acids are polyunsaturated fatty acids where the first double-bond is found on the third carbon from the Omega-end. There are two broad ‘kinds’, namely DHA or docosahexaenoic acid, and EPA or eicosapentaenoic acid which are the long-chain forms and ALA or alpha-linolenic acid which is shorter chain. Long-chain Omega-3 fats are found in fish and shellfish (algae also contains DHA) whereas short-chain Omega-3 is found in plant sources of fat like nuts and seeds.
EPA and DHA are the most potent forms, and in fact ALA must be converted into DHA before it is useful and it takes a lot of ALA to make ‘enough’ as the conversion is highly inefficient – therefore animal sources of Omega-3 are preferable. Vegans may wish to supplement with an algae based product for this reason, though there should be no reason a vegan could not get the minimum required amount of Omega-3 to avoid deficiency, around 250mg combined per day of EPA and DHA, which can be consumed via plant sources in the form of ALA.
Long-chain Omega-3 fatty acids have a huge number of benefits for health, including a reduction in blood triglycerides, an increase in HDL and a very minor decrease in LDL. Omega-3 also seem to reduce inflammation which, if you recall, was a significant factor in atherosclerotic plaque formation due to irritation of the arterial walls. On top of this, EPA and DHA can potentially improve mood and even muscle protein synthesis!
So how much fat should I eat?
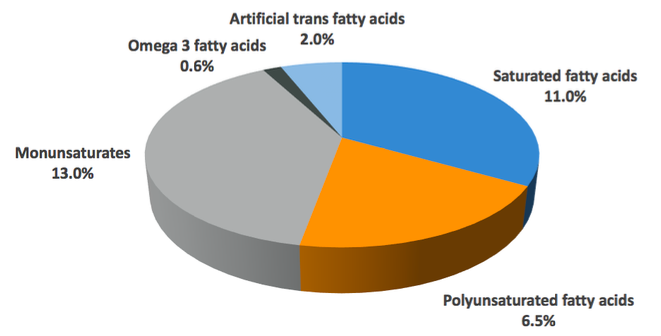
According to the Eatwell Guide, the British recommended intake of fat is less than 35% of total calories, which represents a moderate fat intake essential for a healthy, balanced diet. During times of high energy output the proportion of fat in your diet may reduce due to an increased carbohydrate intake, or you may reduce fat intake during times of caloric restriction in order to maintain a high carbohydrate intake for exercise performance purposes, but this should not drop below 20% as this is associated with impaired sex hormone production and a potential risk for certain vitamin deficiencies as explained in the next module. The Eatwell Guide also breaks this down to recommendations for each different group of fatty acids. Those recommendations are seen below.
Currently the British recommended intake of fat is less than 35% of total calories, and we do not seek to deviate from this. In previous years, it has been postulated that the ideal diet is a low-fat one, but it’s worth noting that this 35% advice would represent a moderate fat intake. The lower-end recommendation is 20%, and this is again something which we fully support. Some dietary approaches may involve higher intakes than this, but for general purposes this recommendation holds water.
Saturated fatty acids should make up to 11% of total calories. As you now know, however, not all saturated fatty acids are the same, and there are some which appear to be more useful than others. The medium-chain saturated fats found in milk fat and exotic oils like palm oil or coconut products as well as those in animal products like beef, seem to be preferable to other saturated fatty acids which you might find in packaged goods, and realistically a good rule of thumb is to get saturated fat in your diet from unprocessed foods wherever possible. If you do this, and don’t over-emphasise one source over the other, it’s unlikely that you will come to any harm.
Polyunsaturated fatty acids should make up around 6.5% of your total calories. Poly-unsaturated fatty acids tend to be found in vegetable oils and some condiments like mayonnaise, but also in grains like oats.
Monounsaturates should make up around 13% of your total calorie intake, coming from sources like avocado, olives and nuts as well as non-hydrogenated butter substitutes/oils made of them.
Omega-3 fatty acids from fish, algae or supplementation should make up at least 0.2% of your total calorie intake. The current literature appears to show that doses ranging from 1-6g of total EPA and DHA are ideal for improving health markers (though around 250mg is enough to avoid deficiency). Supplementation is a cheap and easy way to get this if you would like to experiment with higher intakes. As a warning, do not exceed the maximum studied beneficial dose of 6g, as after a certain amount (not 6g, but there is no need to go higher) your blood will be unable to coagulate properly, which is a major issue. 2-3g per day is a pretty safe, standard range to aim for to get additional benefits over and above deficiency avoidance.
Artificial trans fatty acids found in processed foods, margarines and other hydrogenated oils should make up no more than 2% of total fat intake, but the ideal intake here truly is as close to zero as you can get. Note that there is a very small amount of naturally occurring TFA in some meat products, but this isn’t concerning with any normal level of intake.
Applying this will be explained at the end of this module (because to track and measure that would be just about impossible), but as a last note on fats, remember that most foods contain a mixture of saturated, mono and polyunsaturated fats, so even foods which we might consider to be a source of one may in fact be a source of all three in differing amounts, for example:
- Butter which is considered a source of saturated fat actually contains 54g saturated fat, 19g monounsaturated fat and 2g polyunsaturated per 100g
- Coconut oil is considered a saturated fat source, and it largely is, but does contain 85.2g saturated, 6.6g monounsaturated and 1.7g of polyunsaturated fat per 100g
- Olive oil contains 14g of saturates, 70g mono and 11g polyunsaturates
With all of that covered, let’s move on to our last macronutrient, carbohydrates.