Carbohydrates
A carbohydrate is an organic compound found in the foods we consume (remember, organic only means that they are carbon based at a molecular level). Much like fats they are a bone of contention amongst those in the nutrition field, with many believing that they are the root of all evil and the cause of all weight gain, while the government Eat Well Guide dictates that we should base every meal around them.
Though it is true that those who are overweight eat a lot of carbohydrate containing foods, the idea that carbohydrates cause weight gain per se is not strictly true, as we hope you understood from module 1 of this course. No amount of carbohydrate can cause weight gain if the second tier of the pyramid, calorie balance, is accounted for.
With that said we can state that carbohydrate intake is not essential for survival, and as such it is very easy to reduce carbohydrate intake when you are looking to create a calorie deficit without impairing muscle retention by reducing protein, or overall health by restricting fat by too much. It’s also not to say that eating carbohydrate in place of adequate protein or fat is by any means a good idea.
Carbohydrate’s main job within the body is to provide energy – in fact, when all macronutrients are available your body will ‘burn’ carbohydrates preferentially. You will always be turning over protein, you will always be using fats for cellular function and energy and your body will always be using carbohydrates for something, too, but the ratio of carbohydrates:fat:protein being used at any one time is not static. Exercise changes it dramatically, but at rest the key thing to remember is that your body will burn excess carbohydrate, then it will use excess protein to be converted into carbohydrate to use, and then it will use up fat.
This can often be seen misrepresented in phrases like “Eat fat and you burn fat, eat carbohydrates and you don’t”, but think carefully, do you want to burn fat, or do you want to burn bodyfat?
To burn bodyfat you must release it from fat stores during times of low energy, and the times of low energy are always going to be between meals and while you sleep. If you eat more fat, you’ll be burning more fat, but you’ll also be eating more fat to burn, leaving you at a net zero, and therefore only losing bodyfat if your calories are in line – logical, right?
If you are in a calorie deficit to lose weight, you are by definition eating less overall food. When you eat a meal, the nutrients within that meal must be stored, used for bodily functions or used as energy, and whether that meal was high-fat or high carbohydrate it doesn’t really matter a huge deal for the discussion of weight management – you will either use that dietary fat, use that dietary carbohydrate or use a mixture of the two for immediate energy needs, then you’ll store some of it and then later you’ll start to mine into stored carbohydrate and fat.
Low carbohydrate or low-fat diets both work because calorie balance dictates that fat from adipose tissue will eventually be used as fuel. To assume dropping one nutrient will make a difference is a short-sighted approach and of course, above all, we must remember that macronutrients govern almost everything, but calories are king.
Let us repeat that again, so long as you’re eating in a calorie deficit with enough protein to maintain your muscle mass and health, the ratio of carbohydrate to fat within your diet doesn’t really dictate fat loss for a given calorie intake, and as such your macronutrient intake should be largely down to preference, with respect to the recommendations around the minimum protein and maximum fat intake which we have covered already.
So with that covered, let’s get geeky and learn what a carbohydrate actually is.
Carbohydrate types and understanding the names
A carbohydrate is a sugar molecule or a chain of sugar molecules bonded together to form slightly different chemical structures. The sugars and sugar chains which we usually consume within our diet are part of the saccharide family of carbohydrates (from the Greek Sakkharon meaning ‘sugar’) and they contain carbon, hydrogen and oxygen.
For example, lactose is one example of a carbohydrate molecule, it is the main sugar found in milk and consists of a molecule each of the sugars galactose and glucose bound together.
Because Lactose is made from two simple sugar molecules, it is known as a disaccharide. There are four main categories of sugar, namely:
- Mono: Meaning one, or a simple sugar, such as glucose or fructose
- Di: Meaning two. Again a simple sugar, such as lactose or sucrose
- Oligo: Meaning a few, as they contain 2-9 monosaccharides. These tend to have a minimally sweet taste and are soluble in water while being indigestible to humans and therefore providing some benefits which we will discuss later in this course. One example is the collective fructo-oligosaccharides derived from onions or Jerusalem artichokes
- Poly: Meaning ‘many’, as polysaccharides have 10 to several thousand mono-saccharides within them. These are more complex sugars, often tasteless or slightly sweet and insoluble, such as starch from potatoes or cellulose, a form of fibre found in most vegetables. These are typically referred to as ‘complex carbohydrates’, though fibre is often taken as a separate topic
What is a sugar?
Glucose is the most important form of sugar/carbohydrate in nutrition as it is the main molecule used for energy transfer within your body.
Indeed, when we talk about blood sugar, we are referring to the glucose content within your blood. Photosynthesis in plants, which is the root way which all lifeforms get energy (plants convert sunlight to energy and store it, then get eaten by animals which store it, which then gets eaten by animals that store it) stores energy in the form of glucose, meaning this one sugar is the basis for all energy usage in all life forms that we know. While animals also use fat as fuel, most of that fat is created in animals after they consume glucose. Some plants (mostly the seeds) also contain fats, but again these are synthesised using glucose. In summary, glucose is important.
Glucose linked in long-chains forms glycogen, which is the name for the carbohydrates that get stored in your body. By linking it in another way we get starch which is the most widely consumed carbohydrate in our diets (found in rice and potatoes, amongst other things) and by linking it to another sugar, fructose, you get table sugar. But what is it?
The formula for glucose is C6 H12 O6 meaning 6 carbons, 12 hydrogens and 6 oxygens. You’ll notice that this means we have carbon and 2 hydrogens for every oxygen, and this is where the name carbo-hydrate comes from. Drawn as a stick diagram it looks like this:
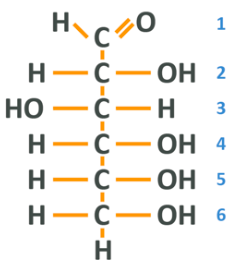
Note: We have a familiar carbon chain where each carbon is ‘full’. At one end, we have what is known as an aldehyde group which is simply a carbon, double bonded to an oxygen and also bonded to a hydrogen. We then have 4 carbons with a hydroxyl group which you will recall is an oxygen and a hydrogen, and another hydrogen, then a final carbon with a hydroxyl and 2 hydrogens. We can number the carbons from the top 1-6, which will be useful in a second.
Now, this is glucose in its simplified linear form which allows for analysis of the molecular components, but in nature glucose is not linear, it is hexagonal. The true shape of glucose more closely resembles the below:
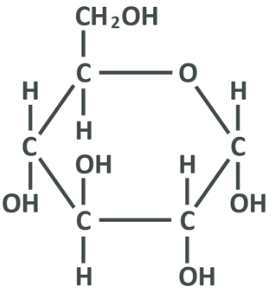
Here the carbon at 3-o’clock is the carbon 1 from the linear picture, and protruding from it you see the rest of the aldehyde group (OH and a separate H), and the top left protrusion is in fact the 6th carbon from the linear picture with the OH and 2 separate carbons. Here, too, the oxygen attached to carbon 5 in the linear picture has lost a hydrogen to bind to carbon 1, and the oxygen from the aldehyde group has lost one of its bonds (recall it was a double bond) and gained a hydrogen. All you really need to remember from this section, is that the carbons in glucose are in a chain and can be linked from 1-6, and in the body and in food the chain connects to itself end-to-end and makes a ring.
The next thing to see here is that the line closest to the bottom is thicker, and that is done simply to give perspective. This is not a 2d circle, the hexagon is lying horizontally and facing away from us, which is key as it shows that carbon 6 and some other atoms sit ‘above’ the glucose while the others sit underneath. This is important and we’ll come back to it later, but just consider that you can look at the linear diagram as a side view.
So that’s a single glucose – let’s introduce another and bond them together in what is known as a ‘condensation reaction’ or ‘dehydration synthesis’ which you have already encountered a couple of times.
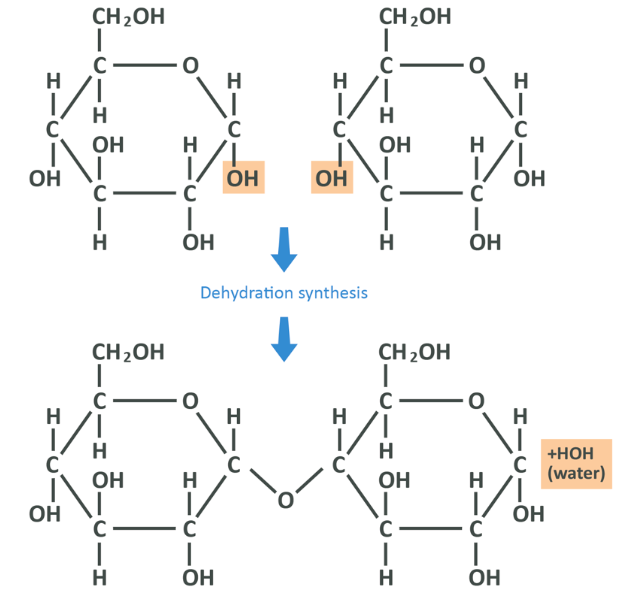
Here you see that the OH group from carbon 1 of the left-hand glucose and the hydrogen from the OH group on the right-hand glucose’s 4 carbon have broken off, and formed a molecule of H20 or water. This leaves the oxygen from the right glucose and the 1 carbon from the left both unstable, and so they bond together (in what is known as an alpha 1,4 glycosidic bond, 1-4 denoting the carbons that have been connected), linking the 2 molecules of glucose into the disaccharide known as maltose. These chains can continue lengthening to create oligo or polysaccharides. This 1,4 bond is the most common link between glucose molecules, though importantly it’s not the only one – this will come up again later.
By linking together chains of monosaccharides we get a multitude of different poly-saccharides, with the type of saccharide being dictated by the chain length and 3D shape. Starch in potatoes as well as cellulose (plant fibre) which gives vegetables their shape and is the primary ingredient in paper are polysaccharides but interestingly so is chitin (kite-in) which forms the exoskeleton of some insects and the wings of flies, and naturally sourced cotton.
One other important monosaccharide is fructose, often referred to as “fruit sugar”.
The chemical formula for fructose is C6 H12 O6 which should look familiar as it’s exactly the same as glucose. In fact, side by side, they look very similar but have distinct differences.
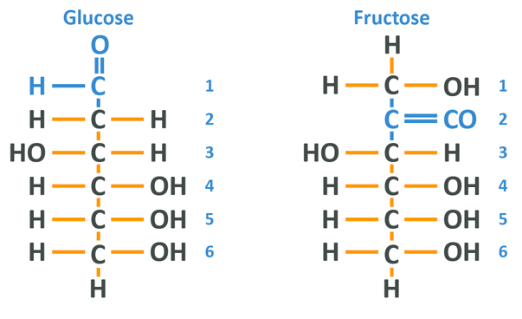
The difference here is that the glucose molecule has a carbonyl group (carbon double bonded to an oxygen) at the 1 carbon whereas the fructose has it on the 2 carbon. Other than that, the 2 are about the same in this format – the key difference comes in when we look at fructose in the cyclical formation which is, like glucose, how it actually appears in nature.
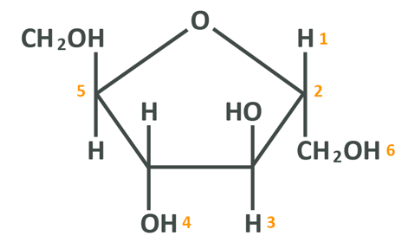
Here the 1 carbon is the one on the far left, and exactly as in the glucose, the double-bonded oxygen becomes bound to the carbon/hydrogen molecule on the 5 carbon. The difference, of course, being that now the double-bonded oxygen is further down the chain compared to glucose. The 2 are very, very similar.
In fact, if the 2 carbon from the fructose bonds to the 1 carbon from the glucose, we get sucrose which is table sugar (this is an alpha 1,2 glycosidic bond).
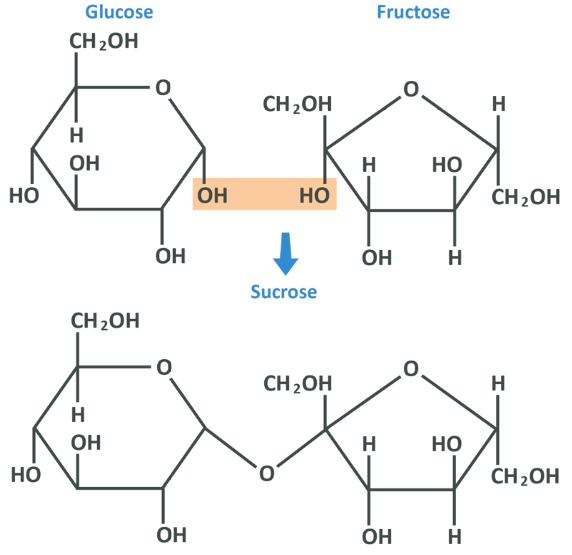
The key thing to remember here is that fructose and glucose are very similar from a molecular viewpoint but are structurally different, and are capable of bonding to each other in more or less the same way as glucose can bond to itself.
Now we know how di, oligo and polysaccharides are formed from monosaccharides, let’s look at how we can digest and absorb them for use.
Starch digestion
To show how carbohydrates are absorbed, it’s best to talk about one of the most commonly consumed carbohydrates in the modern diet – starch.
Starch is made up of 2 different polysaccharides: amylose and amylopectin. Amylose makes up 20-30% of starch and is a long-chain of 1,4 glycosidic bonds between glucose mono-saccharides, and amylopectin which makes up 70-80% has a similar configuration, but approximately every 24th to 30th glucose, a branch is formed when an additional glucose bonds in a 1,6 glycosidic bond as you see below.
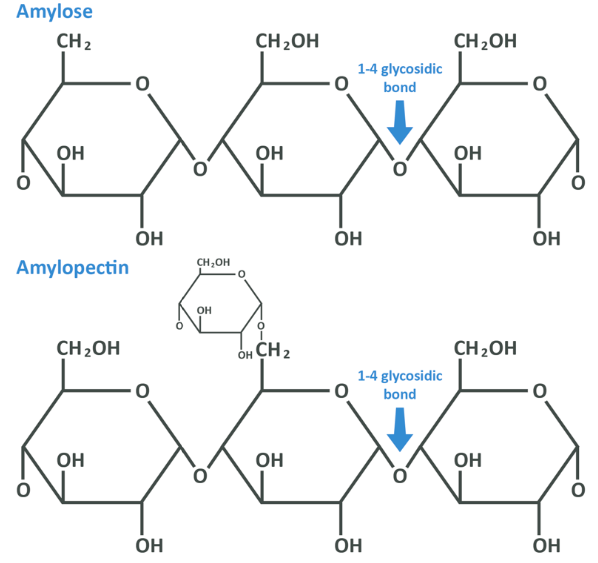
Now, each chain is extremely long. Amylopectin is comprised with between 2 and 20,000 glucose units and amylose tends to be around 300-3000 units. We therefore definitely need to process these before we can absorb them and use them for anything. Remember, it is the simple sugar glucose which we transport in our blood, and here that glucose has been combined into a larger form – by now you should be able to guess that this is a job for our digestive system and, mostly, for enzymes.
When you consume a food containing starch, as for any food the process of digestion begins in the mouth. For protein and fat this process simply involves physical digestion by chewing but carbohydrates have a slightly different fate – your salivary glands produce salivary amylase, which is one of the enzymes capable of chemically digesting the starch polysaccharides. Amylase is capable of hydrolysing (adding a molecule of water to) some of the 1,4 bonds within both polysaccharides. As you learned earlier, the reaction required to join 2 glucose molecules is dehydration synthesis and as such you can think of this like salivary amylase adding that molecule of water back and therefore separating the 2 units again. This is a hydration reaction or hydrolysis.
Salivary amylase is only capable of partial digestion, but its effects are profound nonetheless. As we said above you’ll notice this by chewing some bread for a long time – as the starches are gradually reduced to their constituent glucoses, it will start to taste sweeter.
Next, you swallow and the food travels to the stomach. In the stomach, the acidic environment which was required for pepsinogen to activate and become pepsin denatures the amylase enzymes (as you’ll remember, enzymes are proteins and if their shape is altered they no longer work) and here digestion of the starches pauses. When you are at this point, amylose and amylopectin have been broken down from very long polysaccharides into shorter polysaccharides, some oligosaccharides and a few disaccharides. Of course, as always, the next stop is the first section of the small intestine, the duodenum.
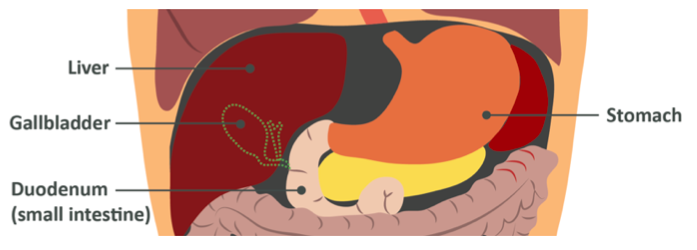
When starch (which is now partially hydrolysed) reaches the intestine, the pancreas releases more amylase (specifically pancreatic amylase) into the area to continue breaking down the 1,4 glycosidic bonds. This breaks the starch down even further into disaccharides and a few very short oligosaccharides.
Recall the billions of small hairs on the walls of the small intestine cells, enterocytes, called microvilli? When the disaccharides and oligosaccharides reach these, they encounter specialised brush border enzymes called maltase and sucrose-isomaltase which finish off the job by respectively breaking down 1,4 bonds between maltose disaccharides left over, and the 1,6 glycosidic bonds located on the branched sections of the amylopectin chains.
What is important to remember here is that starch breakdown starts in the mouth, then is continued in the main part of the small intestine before being finished off by enzymes on the cells lining the intestine. After this entire process, the long-chains of polysaccharides which we consumed are no different to how they would be if we had consumed straight glucose. By the time it reaches your stomach – glucose is glucose.
These glucose molecules are now absorbed through the intestinal wall and into the blood for transport to the liver.
Note: Quick note before we continue – some starch does not fully digest, and therefore is not absorbed. This is known as ‘resistant starch’. Remember that amylopectin which makes up the majority of starch is branched? Well this branching creates a great deal more surface area for enzymes to attack, and therefore makes it easier to digest. Amylose is a little harder to break down and therefore it is hydrolysed more slowly in the small intestine, and sometimes some is missed.
Resistant starches cannot be absorbed and so they pass to the large intestine, the colon. We will return to these in detail in the module on fibre, but consider for now that this is a food from which we cannot extract energy, and which will stay in the digestive system for longer, therefore keeping you better satiated.
Lactose digestion
One other common carbohydrate we consume is lactose, the disaccharide formed between glucose and galactose. Much like starch, it passes through the digestive system but until it reaches the enterocytes it remains untouched – amylase cannot hydrolyse the bonds between glucose and galactose, which is a great example of the specific nature of enzyme action.
Once lactose reaches the enterocytes, the enzyme lactase breaks these bonds and the glucose and galactose monosaccharides can be absorbed. Galactose is mostly sent to the liver and converted into glucose to use just like any other glucose.
Some individuals, however, do not produce as much lactase resulting in the digestive abnormality known as lactose intolerance. Because the disaccharides are not digested properly, they make their way in-tact to the large intestine and ferment, causing GI distress and its associated symptoms. Note that this is very different to an allergy, which is an immune response rather than a gut-focused fermentation and is beyond the scope of this course.
It is estimated that around 5-15% of people with white European descent have lactose intolerance, around 30% of those with Indian descent, around 70-75% of those with African descent, around 80% of those with central Asian descent and up to 100% of people of east Asian descent.
The reason for the differences by ethnicity is interesting, and is more to do with the geographical location of your ancestral roots than anything else. In human beings, the ability to produce the lactase enzyme is almost always present at birth, but diminishes over time. This is because, evolutionarily, mammals stop consuming milk after weaning. At the dawn of the agricultural revolution approximately 10,000 years ago, many humans began drinking bovine milk until later life, and over a very short period our ability to consume dairy evolved (this is a great example of the speed at which evolution can occur with intense environmental pressures) but the uptake of this practice greatly varied by region. In central European areas (especially Scandinavia), dairy consumption is huge, and therefore lactose intolerance in those descended from these areas is very rare. In East Asia, soy-based milks were preferred and so the adaptation to this dietary practice did not occur.
While stomach flu and other stomach issues can temporarily cause lactose intolerance, the idea that you can cause lactose intolerance by abstaining from dairy for extended periods is unsupported in the literature, this can be mistakenly believed because lactase production may naturally decline through life meaning that those who could consume it in their 20’s but do not, may indeed find themselves unable to in their 40’s.
Lastly, lactose intolerance is not a black or white issue, and many who suffer are able to consume a certain amount of dairy before issues arise, and many who do not suffer can encounter problems if they consume truly vast amounts of dairy in one sitting. It is very rare that someone is unable to have any dairy at all, but this should be assessed on a person to person basis. If you do not experience symptoms with a given intake, it can be assumed that this intake is not harmful to you as either your lactase production is sufficient for that small amount, or the small amount of disaccharides which make it to your large intestine do not cause problems.
According to the British Nutrition Foundation many with lactose intolerance can tolerate some amount of milk, yoghurt and cheese (especially low lactose cheese like parmesan). This would also stretch to whey protein where the lactose content is extremely low. When in doubt, however, speak directly to a dietician. This course is not here to diagnose or help manage dietary allergies or intolerances.
Sucrose digestion
The final carbohydrate which we need to discuss is sucrose – table sugar. Sucrose is a disaccharide of glucose and fructose joined together as you have seen.
Sucrose is probably the most commonly consumed disaccharide in the Western World, largely due to its sweet taste. Glucose is not actually that sweet, despite being a ‘sugar’, but fructose binds to taste receptors very well and provides a very enjoyable flavour – leading to sucrose being added to packaged and processed foods, snacks, drinks and desserts the world over.
There are a number of issues surrounding table sugar which we’ll discuss throughout this course, but for now all you need to know is that much like lactose, it reaches the walls of the small intestine intact, but once there it is broken down to its constituent parts by the same sucrose-isomaltase which breaks down isomaltose.
What happens once sugars are absorbed?
Whether you ate bread, sweets, yoghurt or an apple, what you have now are single sugars – mostly glucose and fructose but also others like galactose, ribose and mannose. These last three we will not explain in a huge amount of detail – they have functions and they are metabolically interesting, but to keep this module reasonably readable we will say only that galactose is used more or less in the same way as glucose, practically speaking (we’ve already explained that it is exported to the liver and converted to glucose there), and ribose and mannose as well as a list of other monosaccharides we have not mentioned are used for very different, typically structural functions.
Let’s instead focus on glucose and fructose – the main dietary monosaccharides. We’ll start with how they get from the intestine to the blood:
- In order to reach the blood, monosaccharides are absorbed into the cells on the walls of your intestine, travel through these cells to the other side, and are then absorbed into the blood from these cells, rather than being absorbed into the blood directly from the intestinal cavity. They go across one membrane, across the cell and then across the other
- Sugar molecules are not able to diffuse across a cell membrane by themselves, they need to be helped out by transporter proteins
- The transporters we are talking about here are known as Glucose Transporter proteins (GLUTs), as well as Sodium-Glucose Transporters (SGLTs)
- There are 14 known GLUTs, often expressed at different tissues, for example GLUT1 is found in a great deal of places whereas GLUT4 is expressed near muscle tissues, fat tissues and your heart. You don’t need to know them all, and only 4 will be mentioned in this course. They are incorporated into the cell membrane and allow glucose to cross into the cell, but may not be here all of the time. GLUT4 transporters are usually kept inert inside the cell, and are transported to the membrane to start doing their job when certain signals, which we will discuss, reach the cell from elsewhere in the body
- After you eat a carbohydrate rich meal, GLUT2 transports glucose from the intestine and into the intestinal wall cells
- If there is fructose present, GLUT5 transports it into the cell
- GLUT2 is then responsible for transporting glucose and fructose into the blood from the intestinal wall
So now the monosaccharides are in the blood. What next?
Following transport of glucose and fructose across the cells on the wall of the small intestine, they are deposited into the portal vein which carries them directly to the liver. The liver takes up most if not all fructose, and metabolises it into glucose (along with galactose), meaning that from this point on they are identically managed. It also takes up some amount of glucose while the rest is deposited immediately into the blood.
Note:That because liver absorption of fructose is so effective, there is almost no trace of it in the rest of the body’s tissues.
Some glucose which is absorbed into the liver at this step is used within the liver for energy, some is stored as liver glycogen (we’ll return to what this means shortly) and the rest is returned to the blood as blood glucose or as it is typically referred to – blood sugar.
This is what happens when a person consumes a diet which is not in significant excess of their calorie requirements. We will return later to the fate of carbohydrates, more specifically fructose, if you are in a calorie surplus while consuming excessive amounts of it.
Maintenance of blood glucose levels
After glucose reaches the blood beyond the liver it immediately starts to raise blood glucose levels (not too surprisingly). Generally speaking, your body ‘likes’ to keep your blood sugar levels between 70-100mg/dl, so around 70-100mg of glucose per 10ml of blood. It’s important to hold a certain amount of glucose in your blood because, as we said, glucose is an important energy source – your brain can only use glucose and it needs around 120g per day alone. Your red blood cells must use glucose, too while, as we will go in to momentarily, it’s also an incredibly efficient energy source for almost all of your other cells, too.
If your blood glucose drops too low we call this hypoglycaemia. Due to your brain being literally starved of fuel, you will feel tired, lethargic and confused while potentially being prone to losing consciousness. If your blood glucose rises too high you enter hyperglycaemia and risk damaging your arterial walls (and you now know where that leads) along with your nerves, kidneys and eyes. If this is chronic then this is the condition referred to as diabetes. Homeostasis is the term used to describe the body’s preferred equilibrium, with the maintenance of that equilibrium being a function of homeostatic feedback loops – when you are cold, you shiver and seek warmth to heat back up, and when you are low on energy you feel hungry. maintenance of healthy blood glucose levels is an incredibly important homeostatic function.
To keep your blood glucose levels in check your body obviously needs to be able to remove glucose after a meal and replenish it during periods of fasting, and it does this through virtue of the ‘storage form’ of glucose, glycogen. During periods of feeding and excess glucose, your body is able to store glucose as glycogen in skeletal muscle and the liver, and then during times of low energy availability it is then able to release this stored glucose again. Your liver is able to store around 100g of glycogen, while your muscles can store somewhere in the range of 350-450g.
Glycogen resembles a chain of glucose molecules linked together with 1,4 glycosidic bonds, with a branch every 8-10 glucoses caused by a 1,6 bond – this is very similar to amylopectin in starch, but with a greater regularity of branches. It’s synthesised within cells of either the liver or the muscle tissue after these cells absorb glucose, but it’s somewhat more complex than simply linking them together in basic dehydration reactions. It’s managed by a series of enzymes and inter-linked reactions, which we will spare you the details of so long as you remember the following:
- The process of synthesising glycogen from glucose within cells differs between liver (hepatic) and muscle cells. In muscle cells, glycogen is produced relatively slowly, and once muscle glycogen is full, muscles stop absorbing glucose. In the liver, glycogen production and also glucose absorption speed up as your blood glucose rises in an attempt to keep levels stable even after muscle glycogen is full
Muscle can store around 450-500g of glucose as glycogen and your liver can store around 50g. On top of this, that stored glucose is constantly being used for something. What this should tell you is that you would need to eat a lot of carbohydrate, and total calories, throughout the day in order to maximise total glycogen storage.
Finally, however, it should also tell you that if you do indeed manage to consume glucose (and fructose) above your needs, and maintain a calorie surplus so that the excess sugars have nowhere to go, your muscle will stop absorbing them from the blood, but your liver won’t, and your liver also can’t store it all. This is a problem, and we’ll get back to it at the end of this module.
In summation, under normal circumstances when glucose and fructose are eaten, fructose is converted to glucose and it largely ends up in the blood. The muscles and liver absorb the glucose that they need, and store it as glycogen for later use, with the liver taking priority. Then between meals this needs to be released from the liver to keep your blood sugar levels stable. This is mostly controlled by two hormones: insulin and glucagon, and we will explain everything you need to know about these momentarily.
Carbohydrate in the diet
Carbohydrate is the only macronutrient which is not considered essential, but as already mentioned it would be a mistake to think that this means carbohydrates aren’t important.
In fact, it is because of the crucial nature of carbohydrate that it could be considered non-essential in the first place. Confused? Consider this – a non-essential nutrient is one which the body is capable of making if it needs to. If your body was not able to make glucose out of other things, that could spell disaster during times of famine, and therefore our body (and that of many animals) has developed this mechanism as a failsafe. Without sufficient glucose, your brain will die, your red blood cells will die, and therefore we are able to make enough each day for survival. Your body does this by breaking down amino acids and/or triglycerides in the liver in a process called glucogenesis – you already encountered this somewhat when we mentioned the fate of digested protein.
We don’t just want to survive, though, we want to thrive, and carbohydrate has other benefits.
Glucose is the only substrate that can fuel anaerobic exercise, which is more or less any intense exercise lasting between around 4-240 seconds (there will of course be some variation). Sprinting, multiple-rep resistance training, uphill cycling, crossfit style conditioning and all other forms of ‘short, hard’ exercise will require carbohydrate to be performed optimally. You are of course able to do these things on an extremely low carbohydrate diet, but performance is impaired and fatigue accumulates much faster.
This means is that without carbohydrate, you are going to be unable to perform at your best in the gym, and we don’t really need to tell you that performing some form of resistance training and/or conditioning work (hard cardio) is hugely important for body composition improvement and overall health.
Your carbohydrate need is going to be largely dictated by two things – your athletic endeavours and your overall calorie intake. Because carbohydrate is non-essential, dropping your total intake to quite a low level would not be directly harmful, and therefore carbohydrate is the macronutrient which can be considered the easiest to manipulate for fat loss. After you have determined the calorie intake you are going to be consuming, first work out your protein intake as this is key, then ensure you are eating enough fat to maintain health, and then your carbohydrate need makes up the remainder. This means that as your calories go up, generally speaking your carbohydrates will too – and vice versa. With that being said, it cannot be stressed enough that carbohydrates are an integral part of a healthy balanced diet, for reference the UK Eatwell Guide in accordance with SACN dietary reference values recommends that adults from 19-64 consume 50% of their total calorie intake from carbohydrates.
Additionally, as a final note, if you are inactive you do not strictly ‘need’ carbohydrate to fuel your activity in the same way as a hard exerciser does, you can feel free (and it might even be a good idea) to increase protein and fat to the top end of their ranges, therefore reducing your carbohydrate a little bit. This will likely make very little difference to physiological fat loss rates, but it might make some amount of difference to your hunger and satiety levels, as protein is more filling, and fats tend to offer a pleasant mouthfeel which can reduce cravings.
With carbohydrate discussed, we will now turn to the two hormones which control storage and release of the three macronutrients – insulin and glucagon.
Insulin: An overview
One hormone which you have probably heard of is insulin, often referred to as ‘the storage hormone’. We have explained how glucose is handled within a cell and we may have given you the impression that glucose (and fructose and galactose) are simply ‘soaked in’ to the cells when local concentration is high, and this is kind of true, but not quite. As we mentioned earlier, monosaccharides are not able to cross cell membranes all by themselves and must be transported via special proteins known as GLUTs. A number of the different GLUTs (mostly GLUT1) allows glucose to slowly absorb into the cells in between meals, but then when blood sugar increases and must be absorbed rapidly, something needs to happen. In the same way, as amino acid levels in the blood begin to rise, your body needs to shuttle them to where they need to be (protein manufacturing areas within cells), and this is a job performed by insulin, too.
The first thing that happens when blood glucose rises is that beta cells on your pancreas receive a signal to tell them that blood glucose is rising. When this happens, beta cells release the insulin they have stored within them, and simultaneously start producing more. This is a very similar process to how bile is released into the small intestine; there is an initial release of a product which has been stored in a reservoir and then a gradual continuation as that product is produced effectively ‘to order’. Insulin is a hormone, which means that it is a signalling molecule (a protein) which is transported via the blood to cells in the body a long way from the original secretory glands, then binds to receptors and changes something about the cell. This is as opposed to other signals in the body which may travel via nerves, or travel between adjacent cells.
Insulin has a huge number of functions, including:
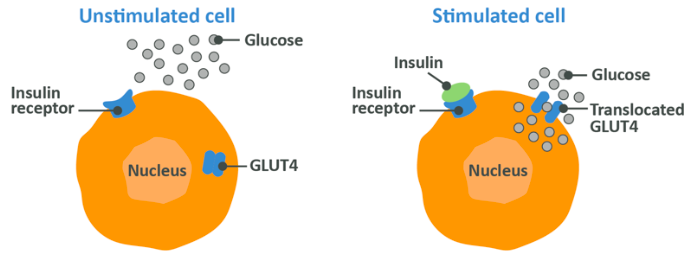
- Insulin causes GLUT4 within muscle cells to move to the cell wall, allowing it to act in those cells to transport glucose across the membrane and into the cell itself. While some amount of glucose absorption can happen during everyday activity due to GLUT1, amongst others, GLUT4 stimulation caused by the presence of insulin in the blood rapidly increases this process
- Insulin stimulates an increase in glycogen synthesis in the cells of the liver, which in turn increases the rate at which the liver absorbs glucose
- Insulin increases protein synthesis, helping to lower blood amino acid levels and increasing the rate at which proteins are made
- Insulin can also stimulate an increase in a process called de novo lipogenesis in the liver. This is the process by which glucose is converted into pyruvate which then ultimately becomes a triglyceride, which can be packaged into VLDL and ultimately stored in adipose tissue. This process is incredibly unlikely to lead to meaningful fat gain, as the amount of triglycerides produced from glucose over the course of a day, even if carbohydrate intake is exceedingly high, is almost certainly to be no more than 5-6g
- Additionally, insulin is able to help triglycerides from the blood enter muscle cells for use as energy, or adipose tissue for storage. It would however, be incorrect to think that insulin is requiredfor fat to be stored. If insulin is not increased due to carbohydrate consumption but fat is consumed and enters the blood, Acylation Stimulating Protein (ASP) in the adipocytes themselves allows fat to be stored
In type 2 diabetes, insulin is not able to increase GLUT4 translocation in muscle cells, or increased glycogen synthesis in the liver, and therefore blood levels of glucose gradually rise and stay elevated. This can cause inflammation and damage to arterial walls, nerve damage and organ damage to boot. When insulin doesn’t do its job, you have problems.
Insulin in effect helps to reduce blood sugar, and it does this through the steps above, as well as by adopting one other means, and that is to counteract the action of its opposite hormone – glucagon.
Glucagon: An overview
Glucagon is the polar opposite to insulin. Its role is to facilitate the breakdown and release of stored energy and it does this via three means. Firstly, it releases triglycerides from adipocytes to allow them to travel to the liver for conversion to glucose, or to muscle cells for direct use in the production of energy. Secondly, it will break down liver glycogen into glucose, and finally it will break down proteins around the body into amino acids, which can be converted into glucose and used for energy.
Importantly, glucagon has very little effect on skeletal muscle or the glycogen stored there because muscle cells do not have a high concentration of glucagon receptor sites. As you can see, if insulin does not ‘stop’ this process from happening – blood glucose will continue to stay elevated, not only due to glucose not being removed, but from additional glucose entering the blood from the liver.
The fasted and fed states
In between meals, glucagon will gradually release fatty acids from your adipocytes and glycogen from the liver to make glucose, which is then deposited in the blood to keep blood glucose levels stable. When you eat, inulin’s first role is to stop this happening, so that more glucose is not deposited in the blood from your body’s stores. Then it gets to work depositing what you have already eaten.
Another issue in diabetes is that this process doesn’t happen. Glucose which you have eaten is not as efficiently absorbed, but moreover, glucose is still being released from the liver, increasing blood glucose to potentially harmful levels over time.
To bring this all together, consider that the body has two key states – fasted and fed.
Fed state: For the first couple of hours after a meal, nutrients will be steadily reaching your bloodstream from the intestines; meaning that as the nutrients are absorbed, blood glucose, blood amino acid levels and triglycerides are elevated. These nutrients will be transported around your body to various cells and tissues which might immediately need them, and what is not needed is either stored (fat and glucose) or converted to something else and stored (protein) to return blood levels to baseline or homeostasis.
Insulin is the primary driver of this. In the fed state, it shuts off the breakdown and release of glycogen, it promotes the formation of new glycogen, it activates protein synthesis in various tissues, and it promotes the storage of fatty acids in either muscle cells or adipocytes. Glucagon is supressed here.
Fasted state: A few hours after a meal (we cannot really give a specific number as it depends entirely on the composition of the meal, but 3-4 hours for a standard sized mixed meal is about right for descriptive purposes), blood glucose starts to drop. The glucose from the meal is no longer present, and your pancreas ‘senses’ that levels are going to reduce to below what they should ideally be.
At this time, insulin secretion is suppressed which then in turn amplifies glucagon release. Glucagon gets to work promoting the release of glycogen from the liver (not the muscle cells as it cannot) into the bloodstream for use around the body during day-to-day activity. A lot of this glucose is used by the brain, and by red blood cells which are another obligate glucose user. At the same time, glucagon encourages the release of amino acids from various tissues and from other proteins like enzymes or hormones – these are then either used in the liver to make more glucose, or they remain in the blood and are used in protein synthesis later. Finally, glucagon encourages the breakdown and release of triglycerides into the blood. The fatty acids can be used directly by various cells around the body (especially your heart) to produce energy, and the glycerol backbone from the triglyceride makes its way to the liver and is used to produce more glucose.
Note: Of course, though glycogen cannot be broken down and released to the blood during the fasted state, you are still able to break it down and use it within the muscle cells which have it stored to perform exercise. This is because the cellular hydrolysis of glycogen and subsequent usage of glucose to produce energy is not dependent on glucagon. Whether your muscle uses stored glycogen or the newly freed fatty acids depends on the intensity of the activity you are performing – whether you are strolling to the kitchen or sprinting for the finish line, your muscle needs to get energy from somewhere, and the reason why it ‘chooses’ one or the other is the subject of a different section.
This is an incredibly brief overview, but we hope it’s provided a pretty simple insight into how insulin and glucagon work together in the fasted and fed states. Insulin isn’t likely to store glucose (or sugar) as fat to any meaningful level, and if it didn’t get released and do its job then glucagon would start to raise your blood sugar levels and kill you – insulin is not a ‘bad hormone’ and increases in it are not something to be avoided.
As a final note here, we hope that this section has shown you just how capable your body is of maintaining homeostasis. If you eat a huge amount of carbohydrate, your body is able to store a lot in the muscle, then what is not stored is cleared up by the liver. So long as, over the course of a day, your energy intake is even, some of that stored carbohydrate will stay stored and some will be released for use and nothing bad will happen. On the flipside, if you do not eat for a long period of time you do not induce hypoglycaemia because your liver glycogen stores will stabilise blood glucose levels, and if this is not sufficient your liver is able to absorb freshly released glycerol from triglycerides in your bodyfat, convert these to glucose and keep everything as it should be.
In fact, it is only after multiple days of fasting that glucose levels would meaningfully decrease, and at this point your body would enter a state of ketosis. This, again, is far beyond the scope of this course, but ketosis is a state whereby there is insufficient glucose to keep blood sugar levels stable and fuel your brain, and as such, the fatty acids released from triglycerides can be used to make ketone bodies in the liver, which can provide an emergency backup. If you wish to learn more on ketosis, we will cover it on our Practical Academy course.
Fructose metabolism
Before we can go further we need to finish discussing the ‘unusual sugar’, fructose. Fructose is unusual because it has a different metabolic fate than glucose and because this different fate can potentially have an impact on our health.
Fructose is found in its free form in fruits, honey and in small amounts in some vegetables. Generally speaking, it’s not likely that you would ever historically have consumed a lot of it. In the modern world, however, fructose intake is significantly higher than it ever has been before due to one factor – sucrose.
As you already know, carbohydrates are absorbed in the intestine and then deposited at the cells in the liver. Some amount of glucose is absorbed immediately for glycogen synthesis but the rest makes its way to the bloodstream. Once there, insulin is released which increases the rate at which glycogen in synthesised in the liver, meaning that glucose is rapidly sucked up into the cells there.
Fructose does not require insulin for rapid absorption, and therefore almost no fructose ever makes it to the bloodstream. This means, incidentally, that fructose has little to no effect on blood sugar and it also does not impact insulin levels. This is why agave nectar, which is almost 100% fructose, is marketed as being ideal for those with poor insulin control.
What happens is that fructose is converted to glucose under normal conditions, but in overfeeding it’s also used to produce triglycerides. In fact, intakes of over around 75g of fructose per day seem to cause worsening of non-HDL cholesterol and total triglyceride levels. With that said, amounts of around 50g do not seem to, which would make at least some logical sense as the liver is able to store at least this much as glycogen anyway (not to say that all of that fructose would be stored, some would be converted to glucose, too).
This is, however, only truly relevant in situations of calorie surpluses. If you are consuming a diet which is in negative calorie balance, though an increased amount of triglycerides may indeed be produced (because your body requires that energy to perform its daily tasks) you will ultimately oxidise more fat than you synthesise, and therefore while losing fat, consuming in excess of 75g fructose shouldn’t greatly impact your health.
Of course, this would entail eating around 150g of table sugar or equivalent (or an insane amount of fruit – a typical 140g apple contains only around 10g fructose) and this is probably a bad idea for dental health and satiety if nothing else.
Summing up digestion, absorption and utilisation
Very briefly, here’s everything we have talked about, summed up simply:
- When you eat something, it is broken down into its constituent amino acids, monosaccharides and triglycerides then absorbed from the intestine
- Monosaccharides and amino acids enter the portal vein and reach the liver
- Fructose and galactose are converted to glucose in the liver, and this glucose (as well as some glucose which was eaten) are either used to make liver glycogen or used to fuel the things the liver needs to do. The rest of the glucose reaches the blood and is sent around the body to be stored in muscle cells or used immediately by various tissues like the brain or red blood cells
- Amino acids are either taken by the intestine or liver for use in protein synthesis, used to make glucose in the liver, or transported around the body for use in protein synthesis
- Triglycerides use the lymphatic system to sidestep the liver, and are transported around the body. They are either broken down into fatty acids and glycerol to use for energy, or stored as bodyfat
- In between meals, these things are then released to use for energy, or used to fuel the cells which have stored them (for example, muscle glycogen isn’t released, it’s used in the cells which have stored it)
- If you overeat, you store more than you release over time and gain fat
- If you under-eat, you release more than you store over time and lose fat
- By increasing dietary protein, you are able to utilise more protein to build muscle, and will not break muscle down so much to provide energy or necessary amino acids
- Certain fatty acids can be harmful if overeaten, and a balanced approach is necessary
- Sugar is not the enemy but it contains fructose and shouldn’t be eaten in enormous amounts – especially if you are eating more than you need to maintain
- Everything is checks and balances, and you can’t really beat the system
We will finish this module by discussing how these substrates are actually used as energy, because they differ in the way that the body can utilise them in a way which explains why we recommend a higher carbohydrate intake in a given calorie intake for those engaged in intense exercise.
Cellular energy production
We will not go into great amounts of detail on this as it’s not 100% relevant for what you will actually do with your nutrition, but it’s important to give you a background understanding as this allows us to understand why things are the way that they are.
When you consume any of the three macronutrients, they can be used within your cells for energy. Your muscle cells use this energy to move, your nerves use it to send signals and your cells use it to make proteins. You basically need it for everything.
- Protein: Can be converted into glucose and used to produce energy
- Fats: Stored as triglycerides, the fatty acids can be used for energy and the glycerol can be used to make glucose, or synthesise more triglycerides
- Carbohydrates: Broken down to glucose which is either used as energy, or in very small amounts converted to fatty acids which can then be used in the same manner as fatty acids that have been consumed
The process by which this occurs is hugely more complex than anything we have discussed so far, but ultimately either glucose or fatty acids make their way to the cells requiring energy – either from the blood or from that cells’ storage sites, and those cells then use internal machinery called mitochondria to convert the substrate into a tiny molecule called Adenosine TriPhosphate (ATP). ATP is the thing which actually ‘gives’ energy to something – and it does it in the following way:
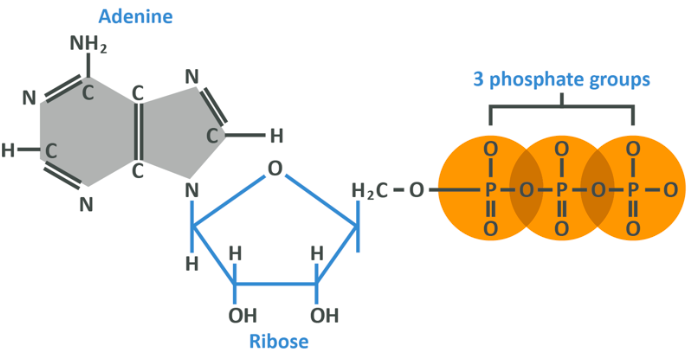
Above you see a molecule of ATP. On the left is an adenine group which is made of hydrogen, nitrogen and carbon. That is linked to a ribose (remember we mentioned this when talking about the different sugars we consume?) which follows a pretty similar configuration, indicating it is a sugar. These two aren’t really so important for this discussion – place your attention to the right-hand side where you see 3 phosphate groups.
Each phosphate group is bound to the next, and it is these bonds which are important.
Within your cell are numerous molecules of Adenosine Di-Phosphate (ADP). You should now know that di- denotes 2, and should therefore be able to deduce that ADP is the same as ATP but with one fewer phosphate group attached.
When cells put glucose or fatty acids through a certain set of reactions, some energy is released, which allows ADP to pick up another phosphate and effectively ‘store’ the energy which has just been produced. Think of ADP like a flat battery – your cells can use glucose or fatty acids to charge that battery by attaching another phosphate.
When your cell then needs the energy, the bond between the 2nd and 3rd phosphate is broken, releasing it, and re-synthesising a flat battery ADP and a spare free phosphate.
The problem is that ATP is difficult to store a lot of. Because of this, your body needs to be able to continually recycle the ADP-ATP-ADP-ATP process, and that means it needs to convert those substrates into energy. It can do this using either glucose or fatty acids, but because glucose is what is used most of the time, we will cover this first.
Glucose is used for cellular respiration, which is a 3-stage, incredibly complicated process starting with glycolysis, moving on to the Krebs cycle and finishing with the electron transport chain which converts one molecule of glucose into up to a maximum of 38 molecules of ATP (though realistically it’s going to be closer to 30 or so). Don’t worry, we are not going to go through all of this in detail, but we will be giving you a brief overview of it so you can understand where things are.
Note: You can also produce ATP using one other thing – the PhosphoCreatine Energy System. This is the ‘fastest’ means by which your body replaces ATP, and is therefore used for intense, often maximal effort such as exertions lasting less than 5 seconds or so. This would be hugely useful for powerlifters, throwers, jumpers, sprinters and athletes involved in martial arts or other combat sports. In order to replace the third phosphate, your body is able to ‘steal it’ from a molecule of phosphocreatine which is stored within muscle cells and therefore does not need glucose.
Muscle cells will always have some amount of phosphocreatine (creatine bound to a phosphate) but those who supplement creatine will have a larger amount. This is the primary means by which creatine can improve performance, and will be discussed in more depth during the module on supplements. After this, we need to consider respiration.
Respiration (aerobic)
The first stage of respiration is glycolysis, the breaking down of glucose which happens within the cytoplasm (cytosol – the liquid interior) of the cell. The first stage involves simply breaking down the 6-carbon chain of glucose into 2 3-carbon chains called pyruvates. This is an anaerobic process, meaning that oxygen is not required to help it – this will become important later on.
At this stage, 1 glucose and 2 ATP’s get used up to produce 4 ATP’s and 2 pyruvates. As a side product, free hydrogens are released which could raise the acidity in the cell and must therefore be ‘cleaned up’. This cleaning up is done by molecules called NAD+ which are free in the cell, which collect the hydrogens to become NADH which then enters the mitochondria. In short, the first stage can be summed up as so:
- 1 Glucose + 2 ATP + NAD + = 2 Pyruvate + 4 ATP + NADH
The pyruvates are then oxidised (meaning that this reaction needs oxygen) into acetyl CoA. This acetyl CoA then enters the mitochondria and is used to produce 2 more ATP’s in the set of reactions known as the Krebs cycle. The Krebs cycle also produces more NADH and some FADH (a similar molecule, for our purposes here, consider them both ‘electron carriers’) which along with the original NADH are sent into the next set of mitochondrial reactions called the electron transport chain.
So far we have produced 4 ATP’s and around 10 units combined of NADH and FADH which have been sent to the electron transport chain. If the above made no sense, just remember that one glucose leads to these things.
The 10 NADHs or FADHs which have been sent to the electron transport chain are finally used to produce up to another 34 ATP’s – the bulk of what the mitochondria can produce for a single glucose. Like the Krebs cycle, this stage also requires oxygen.
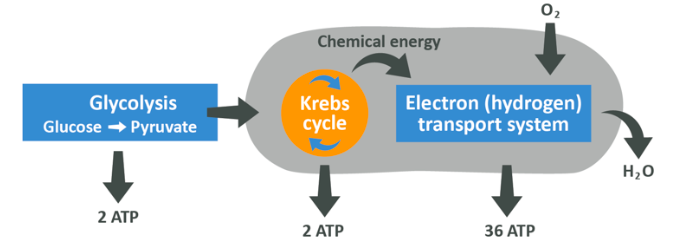
On the left you see the initial glycolysis which gives pyruvate, NADH and ATP (remember that to get NADH, the NAD+ which is always present in the cell gets used up). The pyruvate then enters the Krebs cycle in the form of acetyl CoA and releases NADH and FADH as well as ATP, then finally all of the NADH just produced enters the electron transport chain where a ton more ATP is made, also releasing the now unburdened NAD+ to use again.
The net result is up to 38 ATP’s generated.
This is the way in which carbohydrates are used for energy during day-to-day living and low intensity exercise. It’s a high-yield but relatively slow process which demands oxygen to work (which is why you breathe heavily when you run for a long time).
Sometimes, though, you need energy quickly, often faster than you could supply oxygen. This means that energy must sometimes be produced in the absence of oxygen, through what is known as anaerobic respiration.
Respiration (anaerobic)
Anaerobic respiration occurs almost exclusively in the muscle tissue, because it is only likely to be these whichever work in the absence of oxygen. Other tissues will use the above method to produce energy from glucose.
Anaerobic respiration starts off with the same glycolysis process as aerobic respiration, with glucose being broken into 2 pyruvates in the cytosol. This yields 2 pyruvates and 2 ATP, but because there is no oxygen available to oxydise the pyruvate into acetyl CoA, and there is no electron transport chain for NADH to go into, they build-up.
This build-up of NADH means that there is a depletion of NAD+ going on (as NADH is NAD+ with an added H+ or hydrogen ion) which is a problem. If NAD+ cannot accept the spare electrons, glycolysis cannot occur. What happens now is that pyruvate becomes fermented into lactic acid (sometimes known as lactate) by taking on some of the spare electrons from NADH, resulting in there being more NAD+ and re-allowing glycolysis to occur.
In short, glycolysis adds hydrogen electrons to an NAD+ and produces pyruvate. The resultant NADH can then give the electrons back and become NAD+ again, while turning the pyruvate into lactate. This means that glycolysis can repeat over and over. Crucially, this is far less efficient than aerobic respiration per unit of glucose, but it is vital if you are ever going to perform exercise without oxygen. Without this happening, you could not perform well in a sprint or set of bench press.
The lactate/lactic acid which is produced is then able to be re-absorbed into the blood or surrounding tissues, converted back into glucose or other molecules like amino acids, and stored or used as per normal.
Interestingly, despite what you may have believed, lactic acid is not the thing that gives you ‘the burn’. What gives you the burn is the build-up of free H+ ions.
Fatty acid oxidation
The final means our cells can use to produce energy is through fatty acid oxidation which, as you can probably guess, is dependent on oxygen. This is one of the primary means by which your body fuels your day-to-day life, and it is also a useful ‘energy system’ for extremely long, low intensity exercise.
Of the triglycerides you consume, around 95% of the energy is contained in the fatty acids. The glycerol backbone can also either be used to make energy in the liver, or to make glucose to use in the above manner.
As for the fatty acids, recall that the key stage of respiration which produced ATP was the electron transport chain – here we will basically be doing the same thing with fatty acids. The first stage is to oxidise the long chains of fatty acids into chunks which are 2 carbons in length – Acyl CoA. This then enters a cascade of reactions to produce Acetyl CoA.
Now that the fatty acid has been oxidised into acetyl CoA, you may have predicted that the process released hydrogens to be picked up by NAD+ and carried into the mitochondria for use in the Electron Transport Chain (ETC). We can also then use the acetyl CoA in the Krebs cycle to make more ATP and more NADH and FADH to send to the ETC. You should already be aware that this is creating a ton of ATP. In fact, a 16-link fatty acid like palmitic acid can be broken into 8 acetyl CoA’s, and so can be used to produce 107 ATP molecules. This is an incredibly efficient process!
This is precisely why fats have more calories per gram than carbohydrates, but it’s a very, very slow process and so when carbohydrates are readily available, your body will in general prefer to use these.
In summary
When you are at a state of very low activity, or performing extremely long duration exercise, the majority of the energy used by your body is going to come from aerobic respiration or fatty acid oxidation. While some tissues like your heart which can only use fatty acids or your brain and red blood cells can only use glucose, your muscle cells, liver and many other cells can use both. During day-to-day life or very low intensity exercise, your body could use either fatty acid oxidation or aerobic respiration to fuel what it is doing, and the one it chooses primarily depends on your diet.
If you consume more glucose, you will use more glucose. If you consume more fats, you will use more fatty acids.
During exercise, the primary fuel for muscle cells will always be glucose, though at very low levels fatty acids can be used too.
So… how do we gain and lose fat?
As we have repeated over and over, overeating calories causes fat gain. You may or may not have noticed, however, the specific nutrients you consume which provide those calories are not equally likely to be stored as fat.
Protein which is consumed in excess of need is converted to glucose to use as energy (or store as glycogen to be used for energy later) and some amount of it can be processed and used for energy directly. Glucose can be used or stored as glycogen to use as energy later, while a very, very small amount can be converted into fatty acids either in the liver or in the fat cells, and triglycerides can be stored directly in fat tissue.
This could lead you to think that a low-fat diet is the way forward, and that eating as much carbohydrate and protein as you like won’t lead to fat gain, but you would be wrong. The simple way to put this is that your body and the systems it uses to process nutrients for energy production, storage and survival are way more complicated than that, and there is almost always a feedback loop or other mechanism which, no matter what you eat, brings you at least 95% of the way back to the ‘calories in vs. calories out’ approach, when fat loss or gain is the concern. If you overeat on a diet which is almost entirely coconut oil, butter and lard, you’ll gain fat. If you overeat on a diet which is almost entirely rice, potatoes and pasta, you’ll gain fat, and of course you’ll gain fat in the middle ground, too.
The mechanism for storing that fat, as in where the stored fat itself actually comes from may differ, but the end result is always the same, and that’s all you really need to remember. If you ever hear that X food (for example, pasta or cheese) is more fattening than another food, that claim is wrong. If you ever hear that X nutrient (for example, sugar, fructose, or fat) is more fattening than another nutrient, then again that claim is pretty much wrong too.
Though we will concede that an extremely minor difference to fat gain would be seen if one diet was very high-fat and the other very high carbohydrate with both having the same calorie load, in that the fat would be easier to store. This would have to be an extreme diet which was artificially low-fat or carbohydrate, created with refined foods, and it’s not really that practically useful. For fat loss, there would be no difference at all.
The point remains, that although you can’t store carbohydrates or protein meaningfully as fat, you can certainly gain fat if you overeat them and calories are always king. The long answer as to why you can gain fat while eating macronutrients which can’t really be converted into fat is as follows:
- As you know, weight loss or gain comes down to energy balance. If energy in > energy out, you’ll gain weight. This can then be broken down further into nutrient-specific balance. If fat in > fat out, you’re gaining bodyfat, and therefore fat gain can be considered as a positive fat balance
- Positive fat balance is achieved when fat intake exceeds fat storage, and ultimately that comes down to energy balance. When you consume fat in excess of need, what is not oxidised is then stored immediately in either adipose tissue, in small amounts in muscle tissue and potentially around the organs. When you consume carbohydrate and/or protein, the amount of fat which you oxidise will be reduced accordingly
- Importantly, though, if you are eating a certain amount of calories, the amount of fat you eat will be reduced as the amount of carbohydrate/protein you eat increases. If you overeat fat, you gain fat because you store that fat – but if you overeat carbohydrates you gain fat still because you store the fat which you consumed
- After a high-fat meal, you oxidise the fat in the meal and store any remaining fat
- After a high carbohydrate meal, you oxidise the carbohydrates in the meal and store any remaining fat
Regardless of which way you play it, for a given calorie intake, the fat you gain will be the fat you have consumed, at an amount roughly equal to the calorie surplus you have created. This does assume a given protein intake, though. Because protein is used for protein synthesis, and because it requires some amount of conversion before it can be used as energy (and because of a few other hormonal effects), protein intake can indeed affect your body composition changes for a given calorie level.
The same thing goes for fat loss, too, assuming protein intake is set at the right level, the amount of carbohydrate and fat doesn’t really matter outside of exercise performance and overall preference. This is important, though – go too low on fat and you can harm health, go too low on carbohydrates and you can impair exercise performance and overall perceived energy. The balance that is right for you is individual, this is what is key and now we will work out how to find it.
Summing up the theory; estimating ideal daily intake
What we have done here is given you a huge amount of background information to justify the following recommendations. It’s important to know, however, that this is not the end. Once you have these numbers you need to apply them to your own life, and the way to do that will be laid out in the following modules. It could be implied, if we didn’t include this pre-amble, that the way to eat which is the most health-promoting, would be to weigh and measure all of your food while paying close attention to the labels, and then track this to make sure you are reaching the ‘correct’ intakes for each nutrient, but that may not always be the case.
Of course, many people do indeed track their macronutrient intake and we will discuss this in later modules because doing so is effective as a tool and invaluable as an educational process, but that doesn’t make it the only way. There are methods of ensuring you get as close as is practically relevant without weighing anything, so please don’t feel overwhelmed at this point.
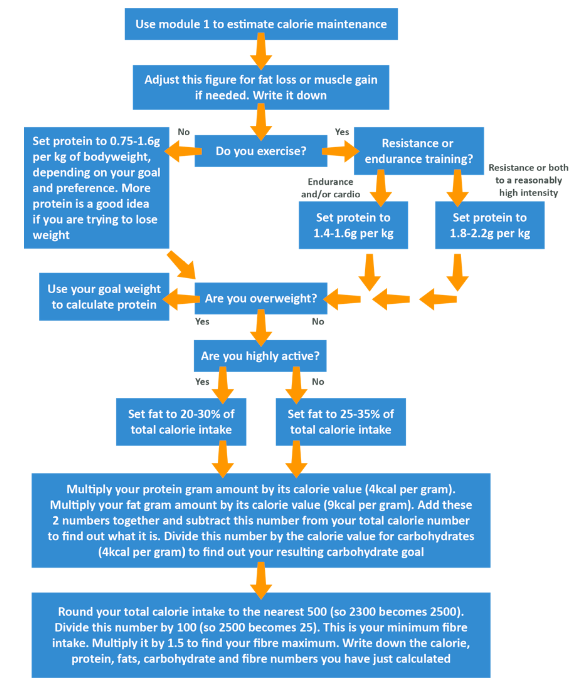
In module 1 we showed you how to estimate your calorie needs. Please use the below to now convert that into your macronutrient needs. The steps to take are in order, but the following flowchart should make this a little easier to work out.
- Calories: As per module 1
- Protein: 0.75-2.2g per kg of bodyweight or ‘goal weight’, from high quality sources wherever possible, broken up over 2-5 roughly even meals per day, with at least one of these somewhere within 2 hours pre and post-workout
- Fats: 20-35% of total calorie intake, split evenly between saturated, mono-unsaturated and polyunsaturated fats, ensuring at least 6.5% of your total calories are polyunsaturated fats and ensuring you get sufficient Omega-3 fatty acids (we recommend at least 1, but ideally 2-3g)
- Carbohydrates: The remainder of your calories, with ideally no more than 50g coming from fructose
- Fibre: 10-15g per 1000 calories consumed. This is the first time fibre has been mentioned in this module and this recommendation as well as the role of fibre will be explained later in more detail, so please take our word for it for now
Confused? Use these steps…
As a baseline, the UK Eatwell Guide recommends the following for a typical man and woman, both relatively sedentary:
| Men | Women | |
|---|---|---|
| Energy | 2500kcal | 200kcal |
| Protein | 55.5g | 45g |
| Carbohydrate (at least) | 333g | 267g |
| Fat (less than) | 97g | 78g |
| Fibre | 30g | 30g |
An example of how this looks can be found in the Foundation Academy video content associated with this module. In the next module, we will turn our attention to micronutrients, the nutrients which we consume in very small (micro) amounts.