Introduction to hydration
What we will now turn our attention to is the thing which makes this transport, absorption and metabolism possible, the aqueous solution in which it all happens – water.
Water is essential for life, and maintaining hydration is important for physical and mental performance. The human body is largely made of water and generally the percentage ranges from around 75% in babies to 60% in adults, and slightly less in the elderly. This of course fluctuates due to certain hormonal conditions, hydration statuses and stages of the menstrual cycle.
Although we can live for up to 50 days and sometimes even longer without food, without water we will survive only a few days even in a cool climate. This is because bodily water is lost every day and without replacing it, the body is unable to perform its normal functions. The water your body stores is the medium in which the majority of nutrients, both organic and inorganic, are absorbed or transported to cells. Then when those nutrients are absorbed into the cell they are taken into the water-filled cytoplasm and involved in various metabolic processes which, themselves, require water to properly occur. No water, no life.
The body isn’t just a homogenous sack, though, so we cannot just think of water as being ‘in the body’. Where your body’s water is, can be just as important as how much there is.
Water within the body can be considered to be in one of two separate compartments. The intracellular water is held within cell membranes and the extracellular water is, as you would expect, found elsewhere, outside of cell membranes. Around ⅔ of the water within your body is intracellular and the rest is found either within the plasma (in your blood) which transports things around your vascular system or the interstitial fluid which includes the water which surrounds all bodily cells and fills the lymphatic system. The lymphatic system is a complex network responsible for facilitating the passage of nutrients and metabolic products in between cells as well as helping move metabolic by-products to areas where it can be secreted. You may have heard of lymph nodes during medical examinations – these are areas where lymphatic fluid collects before draining into the urine or other areas for excretion.
Plasma volume is the amount of water which is held in your blood. As it increases so does your total blood volume and it is for this reason that hydration and blood pressure are closely and crucially linked.
To maintain optimal health, it is not only necessary to ensure that total water content within the body is maintained within given parameters, it’s also valuable to ensure that the water is in the correct places. The maintenance of this balance involves a few different systems including your heart/vascular system, brain/central nervous system, your kidneys/ renal system and your lungs/respiratory system.
Maintaining fluid balance
In terms of what you as an individual can do, water balance is maintained for the most part by consuming water, water based beverages and water containing foods such as fruits, yoghurts and some vegetables. This is important because every day you lose a significant amount of water even without exercising or profusely sweating.
During a typical day at a normal, comfortable temperature, a 70kg individual can lose around 1,400ml of water through urine, 100ml in sweat, 200ml in faeces and up to 600ml in your breath.
In day-to-day life, your hydration status is regulated very well by the sensation of thirst, which exists as a homeostatic mechanism, driving you to seek fluid. Later we will make recommendations for ideal water intakes, but for now let’s explore what we really mean by hydration and water balance because as you will soon see, to be optimally hydrated is not simply a case of drinking a lot of water all of the time.
Osmosis and filtration
Two of the primary means by which your body maintains proper balance between the water in your blood and the water surrounding your cells are osmosis and filtration. This is important because if there is too much water in your blood then your blood pressure will rise which can have serious consequences. On the other hand, if there is too much extracellular water you will have unpleasant subcutaneous water retention and this can lead to damage in some tissues.
Note: As a quick primer before we continue, a solute is something which has been dissolved in a liquid, like sugar in water, as opposed to just mixed in like sand in water. Solute concentration simply denotes the amount of a given solute in a certain amount of liquid. For example, you can mix 1g of sugar into 100ml of water and have a comparatively low concentration solute, or you can mix 70g in and have a thick, syrupy, comparatively high concentration solution. Note that the concentration is specific to the solute, however, so while this mixture is a high sugar solution, it is a low salt solution.
Osmosis
There are many membranes within your body which are permeable to water-based solutions, with cell membranes being by far the most common.
The cell membrane is the outer surface of the cell which allows it to maintain a specific internal environment which is optimised for its own function – consider this in the same way that a fish tank keeps the internal environment ideal for fish life by separating it from the external, air filled environment which isn’t conducive to fish’s survival. Cell membranes are permeable to water and many solutes, but this permeability is selectively controlled – they ‘get to decide’ what goes in and out. This means that cells which are permeable to water can maintain a different concentration of solutes on one side to the other and this creates what is known as an osmotic gradient. When this happens, there is a net movement of water between the high water (low solute) side to the low water (high solute) side. This can be considered like a process of diluting the side which has the high concentration.
This is called osmosis, and the difference between concentrations of a given solute on one side of a membrane vs. the other can be calculated as the osmotic pressure (expressed as mmHg, or millimetres of Mercury which is the manometric measurement of pressure). We won’t go into specific osmotic pressures because they aren’t needed, but it’s an important concept to grasp.
Note: When a membrane is freely permeable to a solute (as in, it just goes in and out in this case without the cell being in control), it does not count towards the osmotic pressure of a given solution. This is because rather than water being forced to rush from one side to the other, the solutes themselves can evenly disperse on either side. As an example, urea (which is a waste product of cellular processes) will therefore be found inside cells at a higher concentration than the surrounding water, and is able to cross a membrane easily and disperse, but many proteins are not, meaning that the latter does but the former does not contribute to an osmotic pressure.
You may have heard the words hypotonic, isotonic and hypertonic before. In the context we are speaking about, a hypotonic solution has less solute than the environment within a cell, an isotonic one is the same and a hypertonic one has more. Here’s a handy image to help you conceptualise what we are talking about.
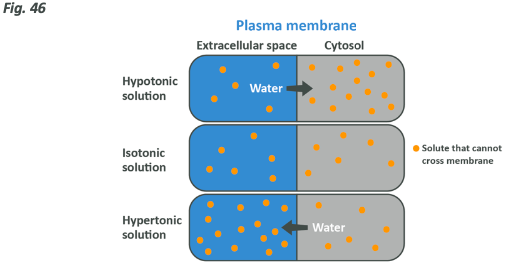
The role of sodium and potassium in hydration and osmosis
Sodium and potassium are two inorganic molecules which carry a positive electric charge. Because of this charge they are often referred to as electrolytes (they aren’t the only ones but are the most important for this discussion).
Sodium and potassium can pass freely across a cell membrane, but the cell then ‘takes control’ of the relative internal and external concentration of each for a very particular and important reason. Each cell is equipped with millions of sodium-potassium pumps which are small proteins on the cell membrane, able to pump 3 molecules of sodium out and 2 molecules of potassium into the cell repeatedly to maintain a given ratio – very high intracellular potassium and very low intracellular sodium with the extracellular environment reflecting the opposite. This ratio creates an electric charge necessary for a variety of functions, but also creates an osmotic pressure. The osmotic pressure in each direction must be kept equal, however, or the cell itself would either shrink or explode because of water rushing out or in respectively. This means that your intracellular and extracellular environments must be fine-tuned to maintain a given ratio of potassium and sodium, and in a broader sense it means that there is an optimal level of intra and extracellular water.
It can be considered that sodium is the key extracellular electrolyte responsible for extracellular water levels, and potassium is the key intracellular electrolyte responsible for intracellular electrolyte levels. If the body’s potassium stores dropped leaving the cell with less potassium than it should have, then the osmotic pressure would result in the cells becoming dehydrated and your body would need to remove sodium to address the balance. The opposite is true too. If sodium levels dropped too low then the cells would be prone to hyper-hydration so sodium levels would need to be increased (this can happen thanks to stores on your bones, or by salt-cravings) or potassium levels reduced.
As you can see, balance between potassium and sodium is just as important as the balance between water intake and excretion.
Filtration
For water to leave the plasma and enter the interstitial fluid it must leave via capillary beds.
Note: Remember your heart pumps blood out through arteries to the body. The blood travels through these arteries, into smaller arterioles and finally to minuscule capillaries which spread out over tissues to maximise the surface area which can supply nutrients. This spread-out area is called the ‘capillary bed’.
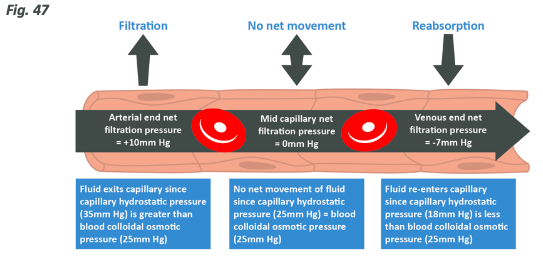
Capillaries have a very thin, permeable wall and so things diluted in the blood can pass out of them quite easily, and things can also be diluted into the blood from the tissues at this point before being passed to various areas in the body which excrete them.After the capillaries have been in contact with their target peripheral tissue, they merge again into venuoles, then veins, then the blood returns to the heart.The pumping of the heart creates hydrostatic pressure (expressed as mmHg also) in the capillaries carrying blood to tissues, and this means that at the arterial end of the capillary bed the fluid is forced out, effectively increasing interstitial water. Because red blood cells and some other proteins necessary in blood are comparatively large, they are unable to leave the capillaries, meaning that only the water and smaller solutes therefore increasing the protein concentration within the capillary (there is less plasma water left in the capillary, so the concentration is higher). Think of this like a hose with a hole in – if you narrow the end of the hose, the water comes out of the hole at a higher pressure. At the other end of the bed, closest to the veins, the hydrostatic pressure is less as a lot of liquid has been removed but concentration within the capillary is now high, which will then cause water to re-enter the vascular system via osmosis and be returned to the heart.
Blood reaches the bed at high pressure and water leaves. At the other side of the bed the pressure is less, but the concentration gradient is large so water re-enters.
As you may have already deduced, the amount of water which travels from one side of a membrane to another is determined by the cumulative pressure between all different osmotic pressures and hydrostatic pressure. The pressures may come from both sides of the membrane, and water will travel in the direction where the pressure is most strong.
How this works in a capillary bed is summed up in the picture below.
The role of the kidneys
Of all the organs and bodily systems involved with fluid and electrolyte balance, it is by far the kidneys that play the largest role.
The functional part of the kidney is a small yet complex structure known as the nephron, with each kidney having somewhere in the range of 1-1.5 million, of these units located along the outer edge of the main kidney body.
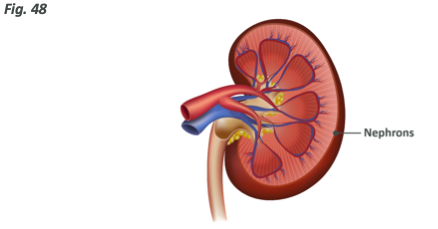
Each nephron (see previous diagram) is broken down into five compartments:
- Bowman’s capsule
- The proximal convoluted tubule
- The loop of Henle
- The distal convoluted tubule
- The collecting duct
Note:Proximal means closest to, distal means furthest from, here these terms denote distance from Bowman’s capsule.
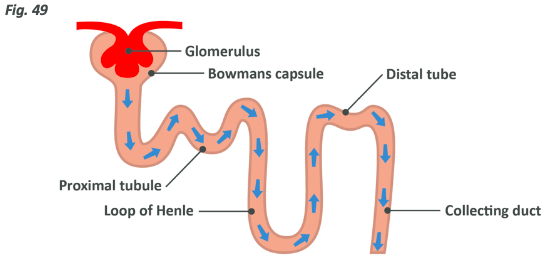
Around 25% of the blood pumped from the heart is sent directly to the kidneys, which should show you how important this process is, seeing as the kidneys make up far less than 1% of the body’s actual weight. This blood reaches the nephron via the capillaries which converge into a complex referred to as a glomerulus.
Glomerular capillaries are a little different to the above capillary beds, which would be more typical in muscle tissues or the lungs for example. In the glomerulus both sides are arterial with an afferent arteriole carrying blood in and an efferent arteriole carrying it away which is smaller in diameter. This setup functions to keep the pressure in the glomerulus around three times as high as it is in other capillary beds, which is key, as around 130ml of blood per minute is filtered through the kidneys, or around 187 litres per day. Because of this pressure, the filtrate which leaves the blood doesn’t get re-absorbed at the other side of the capillary bed but instead makes its way through the nephron for processing.
Of course, you don’t urinate 187 litres of water per day, and that is due to the nephron’s ability to re-absorb water and solutes. At various points through the tube, capillaries which surround it will re-absorb water and solutes via osmosis and put them back into the bloodstream for use, so only around 1400ml of water per day is filtered from your blood and removed. After they have been re-absorbed, the plasma and solutes dissolved in it simply return to normal blood flow.
Blood goes to the nephron, is non-discriminately filtered and then the things that the body needs are re-absorbed with the rest being urinated out.
In fact, it is by altering the rate at which electrolytes and water are re-absorbed in the nephrons that your body can precisely alter the amount of water and electrolytes which become present in the plasma. This is how your body regulates your blood pressure and is also how you maintain proper fluid balance between intra and extracellular compartments.
As a final consideration, it must be remembered that when sodium is being re-absorbed from the nephron this necessarily increases the concentration in the local capillaries to a higher level than that within the nephron itself, and vice versa when sodium reabsorption is restricted. This creates an osmotic pressure which impacts the way that water travels across membranes.
In very basic terms, as far as reabsorption from the kidneys goes, water follows sodium and if sodium reabsorption is increased or decreased, so is water reabsorption to suit. We will come back to this.
To summarise:
- Your blood is full of solutes, most importantly for this discussion sodium and potassium, but also proteins like red blood cells and white cells, amino acids, fatty acids, glucose and a ton of other things
- You drink water and it is absorbed into your blood, increasing plasma water levels and blood pressure, and decreasing relative plasma solute levels
- This plasma is sent to various capillary beds around the body in the blood. The plasma water and solutes (but not the larger proteins) are forced through the capillary walls and ‘bathe’ the cells nearby with water and nutrients such as glucose and triglycerides
- Due to osmosis, some solutes will enter and others will leave the cells. If the solution within the cell is at a higher concentration than the solution in the interstitial fluid, some water will also enter the cell. If the opposite is true then the cell will lose water. This is how cells stay hydrated
- Water is taken up from the interstitial fluid (along with any waste which the cells have secreted into it) back into the plasma at the other end of the capillary bed and sent back to the heart
- Being that a certain water level within every cell is ideal, we should therefore be able to deduce that the amount of water and solutes in the plasma must be kept within certain parameters. If blood concentration becomes too high because there is too little water or too many solutes, the cell will dehydrate and if the opposite is true it can burst
- The kidneys can excrete certain amounts of water, or indeed prevent the excretion of water, and the same goes for various solutes, especially sodium and potassium. This keeps the total level of all three tightly regulated
But how does the kidney know what to do? Why does it excrete more or less water when it needs to? And what about sodium and potassium?
Vasopressin
Vasopressin (also known as antidiuretic hormone or ADH) is a hormone released from the hypothalamus which is a gland located in your brain. Much like how bile is produced in the pancreas and stored in the gall bladder for rapid use, ADH is produced in the hypothalamus and stored in the pituitary gland (also located in the brain).
In order for ADH to be released, an increase in plasma sodium concentration must be detected. From an evolutionary standpoint, this makes sense because historically sodium was very hard to come by, and therefore the only reason that plasma concentration would increase would be because of a decrease in plasma water. Unfortunately, with a healthy plasma water level this effect can also be mimicked with an excessive sodium intake. Regardless of why it occurs, plasma sodium level increases mean that the extracellular water becomes hypertonic and osmosis then means that the cells which make up the pituitary (specifically the posterior or ‘back’ half) dehydrate and shrink, which then creates a signal to release ADH.
ADH then travels in the blood to the kidneys where it serves two primary functions. First it increases the amount of water which can be re-absorbed from the nephrons by directly increasing the permeability of the capillaries there. To repeat and clarify, vasopressin directly works on how water is re-absorbed. This is distinct from the next system we will talk about.
As a secondary effect, vasopressin is able to increase the rate at which sodium is excreted by reducing sodium reabsorption to some extent, and as a tertiary effect it increases subjective thirst. This is why you feel really thirsty when you eat very salty foods.
The key effect, however, is to increase the amount of water in the blood and in so doing, increase blood pressure. This has historically been a crucial part of survival, but of course in the modern world where an elevated sodium intake is almost the norm this can lead to artificially elevated blood pressure and the associated problems. If potassium intake is also high, and therefore intracellular potassium is relatively high, this effect is somewhat mediated (osmotic pressure inside and outside of the pituitary cells would be even) but regardless, an elevated sodium intake risks activating this system in a way it did not evolve to be activated.
This is not the only means by which the body regulates water and blood pressure, however. Next, we will take an overview of the Renin Angiotensin Aldosterone System (RAAS).
The Renin Angiotensin Aldosterone System (RAAS)
This system works via hormonal action which happens in a cascade. While the main job of vasopressin is to increase the reabsorption of water and its secondary effect is to increase sodium excretion, the purpose of the RAAS is primarily to control plasma levels of sodium. By increasing sodium reabsorption, water reabsorption happens on its own via osmosis.
Recall that glomerular filtration happens quickly because the pressure is higher here than in normal capillary beds, and that this process is so important that 25% of all blood leaving the heart undergoes this process. From these two facts alone, it should be obvious that glomerular pressure is extremely important. It’s so important, in fact, that cells surrounding the afferent arteriole near the Bowman’s capsule of the nephron can detect when blood pressure drops.
When the glomerular pressure drops lower than what it would ideally be, glomerular filtration rate would of course be reduced and this could potentially lead to a build-up of dangerous metabolites or other things within the blood. This needs to be rectified and it’s for this reason that the RAAS kicks in. When pressure drops, the kidney produces an enzyme called renin which enters the bloodstream.
This is the main reason that renin is produced – basically, there’s not enough water, there is a reduction in blood pressure, and therefore this system logically starts to initiate a mechanism to stop excreting water and making it worse. There are two other reasons that the RAAS becomes activated.
- The Sympathetic Nervous System (SNS). The SNS is the nervous system which, briefly, creates the ‘fight or flight’ or stress response to stimuli. When you’re stressed/in danger this kicks in to perform a host of tasks including elevating heart rate, increasing alertness and raising blood glucose in an effort to help you run or stand your ground when in danger. The SNS activates renin release to initiate the RAAS because an increase in blood volume and the narrowing of the blood vessels both result in an increase in blood pressure and an easier transport of nutrients to working muscles. This has large implications for individuals with chronically stressful lives, as it provides a direct mechanism for how stress can lead to increased blood pressure and increased blood pressure, as you have learned in previous modules, can lead to damage in the arterial walls which makes for more efficient binding of LDL cholesterol. This is one of the precise mechanisms by which stress can cause heart attacks
- If a decrease in sodium is detected in the distal convoluted tubules, or an increase in potassium, then renin will be released. This again makes sense because, relating back to osmosis, if sodium levels are low and potassium levels are high, that would create an osmotic pressure that could in fact result in over-hydration of the body’s cells, leading to cell damage
In short, renin release (the first stage of the RAAS) is caused by reduced blood pressure, the SNS during times of stress, by a decrease in plasma sodium or an increase in plasma potassium. It’s crucial to know that these do not all have to happen at the same time, and they don’t cancel each other out, so if blood pressure drops but sodium doesn’t drop (so you’re low on water but not low on sodium) renin will still be released.
As a final note on renin, it’s release is proportional to the magnitude of the driver for release. If blood pressure only drops a little, only a little is released for example. So, what then?
Renin’s role in the blood stream is to activate a hormone precursor called angiotensinogen which is produced by the liver. Angiotensinogen secretion happens all of the time in order to make it readily available but it’s completely inert until renin is released to activate it and make angiotensin 1, which is then converted to angiotensin 2 in the lungs by another enzyme called Angiotensin Converting Enzyme (ACE). ACE inhibitors are medications which can be taken to reduce blood pressure, and this is their mechanism of action (that ACE is produced in the lungs is also why ACE inhibitors make you cough).
Angiotensin 2 has a few different roles. Firstly, it increases vasopressin secretion, secondly it increases thirst sensation and thirdly it acts to constrict blood vessels in order to increase blood pressure. It’s fourth and main role, however, is to cause the adrenal glands (a pair of glands which sit on top of your kidneys) to produce aldosterone. To recap:
- The liver produces angiotensinogen
- The kidneys produce an enzyme called renin which converts this to angiotensin 1. Renin release happens because your blood pressure drops too low in the glomerulus, because your SNS is activated, because your potassium level is too high or because your sodium level is too low
- Angiotensin 1 goes to the lungs and is converted to angiotensin 2 by an enzyme called angiotensin converting enzyme or ACE
- Angiotensin 2 tells the pituitary to release vasopressin, tells you to drink water, constricts your blood vessels and tells the adrenal glands to produce aldosterone
- Aldosterone is the end-product of the RAAS, which has an effect on the nephrons
- In the nephron, aldosterone causes the reabsorption of sodium to increase, thus increasing the osmotic pressure pushing water into the surrounding capillaries and therefore indirectly increasing plasma volume. At the same time, aldosterone causes potassium to be excreted in the urine
- The increase in plasma volume and sodium content then increase blood pressure, which reduces the need for renin to be released, preventing blood pressure elevating higher
Summary of water and electrolyte homeostasis
- The body tightly controls its water balance and thus blood pressure through the renin angiotensin aldosterone system and the action of anti-diuretic hormone also known as vasopressin
- Water homeostasis is largely down to the actions of the kidneys which filter and then re-absorb water and electrolytes from the blood. They re-absorb more of what the body needs and excrete what it doesn’t
- With lowered blood volume, and therefore blood pressure, glomerular filtration is not able to do its job efficiently which is a problem
- With imbalanced electrolyte levels, osmosis can either dehydrate or damage the cells of the body. Cells keep a high internal potassium level and high external sodium level which creates an even osmotic pressure and if this pressure becomes uneven there is an issue
- A reduction in hydration manifests itself in the blood as a reduction in blood volume and decreased blood pressure, as well as an increased concentration of sodium
- When blood pressure drops in the glomerulus (the entrance part of a nephron in the kidney), renin is released into the bloodstream. This can also be released if plasma sodium level drops, potassium level increases or indeed if you are highly stressed
- This ultimately results in angiotensin 2 being present in the blood
- If plasma sodium concentration increases above the level it usually is, either due to increased sodium or reduced water, the pituitary also releases ADH which increases water uptake and sodium excretion in the kidneys
- Angiotensin 2 is vasoconstrictive (causes closing/shrinking of localised blood vessels). It also acts on the filtration rate in the kidneys, helping them retain more sodium (therefore water) and thus increases blood pressure
- Thirst levels increase with angiotensin 2, making you drink
- The net result of the RAAS system and ADH is elevated blood pressure. They are also able to regulate the relative levels of sodium and potassium in the blood to maintain an optimal ratio between them
Reducing blood pressure
Throughout the previous section, we have given the mechanism by which the body increases blood pressure. We have noted that this has historically been incredibly beneficial because throughout our evolutionary past a reduction in blood pressure simply denoted a reduction in blood plasma volume which itself indicated dehydration. As such this increase in blood pressure was an effort to reduce dehydration.
Of course, in the modern world this may not be the case. It’s very possible for sodium levels in the blood to be far higher than potassium levels within the cells, which can lead to a decrease in intracellular water, therefore an ADH mediated increase in blood pressure. If this occurs, 2 peptide hormones called ANP and BNP (atrial natriuretic peptide and brain natriuretic peptide) are released into circulation. These increase sodium and water excretion in the kidney and prevent the release of renin, thus lowering blood pressure. If this system is not able to keep up or an individual is particularly susceptible, however, hypertension can result.
Hypertension (high blood pressure) affects a huge proportion of the modern Western World with around 30% of UK residents having blood pressure which exceeds the current assumed safe limits. Hypertension is, like all health issues, multifactorial with factors leading to it including:
- Increased bodyweight (including large amounts of muscle mass). Increased mass means more blood circulation requirements, which necessarily mean higher blood pressure
- A sedentary lifestyle
- Age (risk increases as you get older)
- Genetics/family history
- Smoking
- Race (Black populations are more likely to be diagnosed)
- Stress
- Drinking too much alcohol
- Too much sodium or too little potassium in the diet
Through a number of different mechanisms, an elevated salt intake can lead to elevated blood pressure and there does seem to be a genetic variance between people. Regardless, a large body of epidemiological and controlled research suggests that an intake of over 2.3g of sodium (around 6g of salt) is correlated to an increased risk of illness. That is not to say that the less salt you eat, the better, though. As you have seen, sodium is a vital part of hydration and in fact this is why it is included in all sports drinks. A minimum intake of 1.5g of sodium has been set by the Institute of medicine, which suggests that rather than worrying about reducing salt completely, people should instead reduce their intake of extremely salty processed foods while feeling no guilt whatsoever in lightly salting unprocessed, home cooked meals.
Similarly, as you have seen during this module, the relationship between potassium and sodium is a close one. In research, when potassium intake is increased, the risk of hypertension and all other negative factors associated with a higher sodium intake are greatly reduced. Potassium is found in abundance in vegetables and fruits (especially bananas) so if you are consuming a diet high in vegetables you are more likely to be able to get away with consuming the higher end of the sodium spectrum. The same goes if you are highly active as you will see below.
As an aside, provided sodium and potassium are balanced and sodium intake is not excessive, sodium should not cause unwanted subcutaneous water retention which is an extremely commonly cited reason for people to reduce sodium to as low levels as possible.
Sodium and athletes
As far as athletes go, insufficient sodium intake is potentially just as big of a problem (if not bigger) than excess sodium intake. A sodium insufficiency is known as hyponatremia. It manifests with similar symptoms to dehydration because, as you know, a decreased sodium level can activate renin release and the subsequent angiotensin 2 which makes you thirsty. That angiotensin 2 can result in the excretion of potassium (via aldosterone), which combined with the decrease in sodium can have extremely wide reaching effects including:
- Nausea and vomiting
- Headache
- Confusion
- Loss of energy and fatigue
- Restlessness and irritability
- Muscle weakness/cramp
- Seizures
- Coma
- Death
Hyponatremia is most often seen in athletes who are performing long-duration exercise while drinking plain water. This causes a loss of sodium through sweat, facilitated by further water intake but not replenished via consumption. Though this course is not a sports nutrition course specifically, it would be unwise of us not to mention this. While we will cover dehydration next in some amount of detail, you do not just lose water through sweat and urine, and if you aren’t consuming electrolytes you can run into a lot of problems even if your water intake is exactly where it should be.
This should also be extrapolated out to the general population. If you drink too much water you stand just as much chance of causing yourself harm than drinking too little. You CAN drink too much water.
Of course, this is not to say that you necessarily need to consume electrolyte supplements or sports drinks and in fact these should only be reserved for those performing very long-duration exercise. Most people can get more than enough sodium through salt consumption and potassium through vegetables and fruits. Another good tactic will be discussed in the final section.
Note: Here we have given the UK Government recommendations along with some notes that active people, or those consuming a lot of potassium may be able to consume a little more salt than other individuals. Those who have impaired renal function are generally more susceptible to increased salt intakes and because elevated sodium intake can increase calcium excretion via the kidneys, those with osteoporosis may also do well to keep their salt in check. Likewise, those with existing hypertension of course must be more careful. If you are within these groups or find that any of the above risk factors apply to you, then we ask that you strictly adhere to the guidelines and seek the advice of a medical professional if you have a clinical reason to think that even this salt intake may be problematic.
Dehydration
Maintaining a proper level of water within the body is vital. Water allows all metabolic processes to occur, allows for the removal of potentially harmful metabolic by-products and environmental toxins, helps maintain your temperature and provides a medium for the transport of the nutrients we need to survive. If the level of water within the blood drops, not only does kidney function suffer, our entire body is unable to function optimally.
While the kidneys will seek to re-absorb as much water as possible, at some point you need to drink because water is lost through various means. If water losses exceed water intake, dehydration will ensue. Dehydration can be classified as mild, moderate or severe as diagnosed by the percentage of water that has been lost.
- Mild:Less than 5% water lost = no clinical symptoms, elevated thirst
- Moderate: 6-9% of water lost = significant thirst, sunken eyes, dry lips/mouth, weakness, light headedness, low blood pressure
- Severe: Over 10% of water lost = significant thirst, rapid heart rate, cold hands/ feet, reduced skin firmness, low blood pressure, confusion
You can also use a urine chart like the below to determine whether you are properly hydrated.
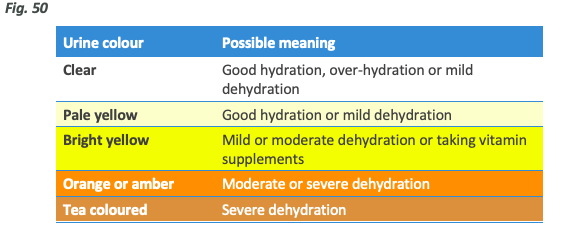
Note: Exercise requires cells to work harder which results in a larger amount of metabolic by-products that must be removed. Even if the plasma level is where it should be after a hard training session (especially an endurance sport), this can lead to darker urine than you would expect simply due to increased solute concentration. As such, post exercise rehydration should be assessed via regaining lost bodyweight and other subjective means such as headaches, dry lips and a feeling of thirst rather than urine colour.
Moderate and severe dehydration obviously severely impact health and daily function, but are unlikely to happen outside of extreme cases including, but not limited to:
- Prolonged exercise in heat
- Prolonged periods where liquids are withheld or unavailable
- Sickness/diarrhoea
- Diuretic use
Mild dehydration can, however, impact your health without necessarily creating any clinical symptoms. Low-level dehydration can lead to poor mental performance, fatigue, perceptions of hunger and some degree of irritability. A dehydrated person is less productive and probably not feeling as good as they could be.
Of course, it can also severely impact on sporting performance.
As loss as small as 2% of body mass can lead to a decrease in performance related to an inability to regulate temperature, increased heart rate and far higher rate or perceived exertion. These issues exacerbate as dehydration progresses until more severe general symptoms appear. It is not possible to train yourself to be able to perform while dehydrated. While mild dehydration will not create permanent problems with your health, it’s best to be avoided.
How can we ensure adequate hydration?
This is the big question. While we have covered a great deal of physiology, biology and theoretical knowledge here, we also need to make some practical recommendations.
As we noted previously, dehydration of up to 5% will not result in clinical symptoms but will result in thirst. We also mentioned in passing the danger of drinking too much water and why this should be avoided.
‘Drink to thirst’ is and always will be the best advice, though this is not a perfect system because people typically will ignore thirst drivers. Though mild dehydration will not cause harm, it can cause hunger pangs and lead to lethargy which aren’t things to actively seek out to say the least. Our recommendations therefore are to consider having a drink alongside your meals, and to keep a small water bottle within easy reach during the day. You don’t need to drink to a schedule or make sure you have X amount during the day, but you do need to cultivate a habit of hydrating when you actually get the urge to do so, rather than ignoring it.
A ballpark estimate of need
While it’s not necessary to stick to a specific amount, it is practically useful to have a rough idea of what you might need. Are we talking 2 glasses or 2 gallons?
The Eatwell Guide recommends an intake of 8 glasses of water per day which offers a reasonable visual/practical idea of what your liquid consumption should look like. To put a number on it, a reasonable calculation is to multiply your weight in kg by 28 and consume this number in millilitres per day of rest. For example, an 80kg individual might require 2240ml or roughly 2.25 litres.
This is of course imperfect and not set in stone. We cannot appreciate your genetic propensity for sweating or your environment, and of course some of the water you consume during the day is contained in your food and not measurable, but this ballpark figure is something which gives you some amount of perspective. If you are exercising that day, consuming water to thirst during the session (consider using a sports drink for intense exercise over 1 hour in duration) is also important.
If you are going to exercise for prolonged period, especially in heat, it can be wise to weigh yourself before and after, then consume the kilograms you have lost in water over the next few hours, alongside a meal which contains at least some amount of salt. If you’re very dehydrated, a sports drink can be useful here.
As we discussed above, tonicity relates to the relative concentration of a solution. An isotonic sports drink is ideal for rehydrating after a highly depleting workout, so this is what we recommend if you have lost a lot of water.
Further information on this topic is beyond the scope of this course. However, if it interests you, we would encourage you to look at our sports nutrition modules in the Body Type Nutrition Practical Academy.
Considerations to make it easier
Drinking water isn’t rocket science, but some people genuinely do struggle to get into the habit of paying attention to their natural thirst. So how would someone increase their fluid intake easily without setting an alarm on their phone?
- Firstly, it’s a good idea to start the day with a glass of water. After sleeping you are mildly dehydrated and this gets you off to a good start. You may find that it helps you wake up, and of course it’s an easy habit to get in to. You can drink a glass while waiting for the coffee to brew
- Add something calorie-free to your water to make it taste better if you don’t really enjoy it. Adding fresh fruit, mint or cucumber to water adds a nice twist, and if you want to, a sugar free cordial works just as well
- In fact, tea, coffee, diet sodas, juices, smoothies, foods and even beer also count to your overall fluid intake. The only things which don’t count are espresso shots or hard spirits as the high concentration of alcohol and caffeine act to increase urination. However, we don’t advocate getting all your liquid from beer
- Always drink during or at least after exercise. If you’re exercising first thing in the morning make sure you are hydrated before going in to the session
- Invest in a water filter if your tap water tastes bad
- Reduce your intake of processed foods to keep your sodium intake within reasonable limits, but don’t think this means you should avoid salting your food. You’re also not going to have a heart attack if you consume a few very salty foods on occasion
- Consider salting food with a Lo-Salt or a similar product, as these replace sodium with potassium. Vegetables salted with Lo-Salt are a great way to boost your intake
In summary, maintaining a proper water balance is vital for maintaining proper health and it can be done very simply – drink when you’re thirsty, don’t over-salt food but at the same time don’t avoid sodium completely and keep an eye on your vegetable intake to make sure your potassium levels are where they should be.