The fat-soluble vitamins
Fat-soluble vitamins are ones which, when eaten in excess, are stored primarily in your liver and fat cells. Because of this, deficiency risk can be considered relatively low, as any days of low consumption can be ‘made up’ by vitamins stored within the body. With that said, of course, those with excessive levels of bodyfat may be at risk of deficiency in at least some cases, because their additional adipose tissue is able to ‘suck up’ some of the vitamins needed in the blood supply. Research has shown that individuals with obesity may be deficient in vitamin D despite a high intake, and this goes away with fat loss – as such, obese individuals especially should pay attention to regular intake, even if the relative risk is still reduced.
Fat-soluble vitamins are, as the name makes obvious, lipophyllic and hydrophobic. You encountered these terms in the last module when talking about fats and cholesterol. Because of this, you may have deduced that it is something to do with the molecular structure of the vitamins, and you would be correct.
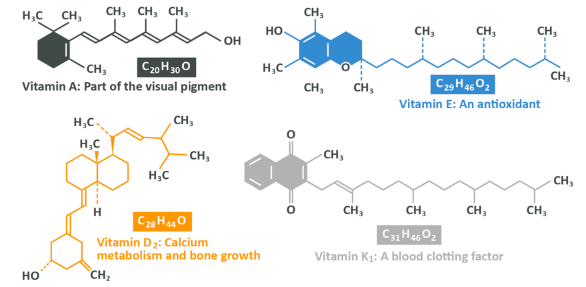
Above you see the structure of the 4 fat-soluble vitamins – vitamins A, D, E and K. They have what should now be a familiar structure involving a long carbon chain, with some carbons being formed into rings. This structure is known as ‘non-polar’ which means that none of the atoms within it have a particular positive or negative electromagnetic charge. Because of this structure, the molecules do not bind well with water and that means that they are not soluble in it or in your blood.
This insolubility means that, much like dietary lipids, once these vitamins are absorbed into the cells of the small intestine they are packaged into chylomicrons alongside triglycerides and cholesterol, then transported around the body for use or deposited back in the kidney for storage. As such, these vitamins should always be consumed alongside some dietary fat (or preferably a mixed meal) so as to facilitate their absorption and transport. If the fat content of the diet is too low, in fact, deficiency of these nutrients can occur even if intake is relatively high due to malabsorption.
Note: In fig. 39 you see vitamins K1 and D2, but there are other forms of these vitamins with slightly different structures and in fact, each vitamin could be considered to be a group of similar molecules with the same (or very similar) functions in the body, rather than one discrete thing – vitamin A is a collection of things, rather than one particular molecule.
If fat-soluble vitamins in excess of need are consumed, they are stored mostly in the liver and fat cells for later release.
Vitamin A
Found in two forms in foods: retinoids and carotenoids. Retinoids are found in animal products, especially liver, cod liver oil, eggs and whole fat dairy, whereas carotenoids are found in red, yellow and orange vegetables including sweet potatoes and carrots, as well as dark leafy greens like kale.
Retinoids are ‘pre-formed’ vitamin A but carotenoids must be oxidised and cleaved in two before they can be ‘used’. While both are absorbed and travel around the body, retinol can be used immediately (and if not, it’s stored in the liver after being delivered there by chylomicron remnants) and carotenoids tend to be stored in fatty tissue in their inert form. If intake becomes low, the carotenoids can then be oxidised for use but this process is quite tightly regulated according to vitamin A levels present in the body. As such, retinol is quite easy to ‘overdose on’ whereas carotenoids cannot realistically become toxic with overconsumption, though they can cause a harmless yet undesirable orange pigmentation to appear in your face, hands and other features, if chronically eaten in excess. If retinoid intake is excessive, toxicity can result in liver damage, headaches and vomiting.
Vitamin A has numerous functions including regulating proper immune function, reproductive function and proper growth of cells, but it’s most prominent function is to do with eyesight. Vitamin A is vital for the resynthesis of Rhodopsin, a pigment on rod cells which are the tiny receptors on your eye. If this becomes chronic, blindness will result, but the immediate symptomology will be ‘night blindness’ where a person struggles to see in dim light.
Deficiency is most likely in populations consuming most of their vitamin A as carotenoids (so, from plant sources) because although around 90% of the retinoids present in a food will be absorbed, this number is far lower for carotenoids, and this is even lower if the accompanying meal is very low-fat. As such, vegans should be very careful to ensure their fat intake doesn’t drop too low, as is common in plant-based diets.
In fact, there is a conversion equation for ‘retinol equivalents’ or RE, which show the difference between 1iu of retinol and 1iu of carotenoids. In order to get 1iu of retinol, you need to consume just under 6iu of beta carotene (the most common carotenoid in commonly consumed foods), meaning that plant based vitamin A requirements are around 6 times that of animal based ones. The recommendations below are given in RE, so a higher level will be required if your only intake is from plant sources.
| Population | WHO AER | WHO RNI | UK RI | Upper limitr |
|---|---|---|---|---|
| Male adults (19-65) | 300ug | 600ug | 700ug | 3000ug per day or 7500ug per week |
| Female adults (19-65) | 270ug | 500ug | 600ug | 3000ug per day or 7500ug per week |
| Pregnant women | 370ug | 800ug | Speak to your doctor* | 3000ug per day or 7500ug per week |
| Lactating women | 450ug | 850ug | Speak to your doctor* | 3000ug per day or 7500ug per week |
- During pregnancy, vitamin A intake is a balancing act. While more is needed to avoid deficiency, which may cause issues either during gestation or during breastfeeding, having too much is just as dangerous. Current recommendations are for supplementation to be avoided during pregnancy, along with liver and liver products, but a blood test will be able to indicate individual needs.
Note: For reference, liver contains around 4,500ug RAE per raw 85g, a 180g sweet potato with skin has around 144ug (1730ug beta carotene) and 100g of carrots has 345ug (4142ug beta carotene).
Vitamin D
Found in two primary forms, D2 (ergocalciferol) and D3 (cholecalciferol). D2 is a plant-origin form which may be consumed in the diet, most commonly from mushrooms which have been exposed to sunlight (or in modern food manufacturing, intentionally enriched via ultraviolet light) whereas D3 can be attained via foods such as milk and eggs, or synthesised by your skin upon exposure to sunlight, thanks to a cholesterol-like precursor called 7-dehydrocholesterol. D2 and D3 are structurally different molecules, but they are metabolised in a very similar way and can be considered to be more or less interchangeable for practical purposes.
While dietary vitamin D can be used to meet needs, the amount of vitamin D present in foods can be relatively low, and as such the main source of non-supplemental vitamin D for humans is that which is synthesised from sunlight. Because of this ability to synthesise vitamin D endogenously, strictly speaking we should not consider it a vitamin at all, going by the definitions established above, but nonetheless it’s a crucial micronutrient.
Upon ingestion or synthesis of vitamin D, it is metabolised in the liver to 25-hydroxyvitamin D or calcidiol, which is in turn sent to the kidneys where is it converted into ‘active vitamin D’ known as 1,25-(OH)2D or calcitriol. The suffix –ol indicates that in fact this active form is a hormone, and in fact vitamin D is a seco-steroid (which simply means that it has a ‘broken’ carbon ring in its structure, one of the rings appears smaller than the others and only has five sides.
Because it’s a steroid hormone, it is able to travel in the blood and enter cells, bind to special receptors and affect the way that DNA code is used. Recall from the last module, that DNA code tells the cell how to make protein, well, vitamin D is able to affect the way that proteins are made. Those proteins then go on to have profound effects.
Vitamin D is required primarily to maintain normal blood levels of calcium and phosphate, which are themselves needed to maintain normal bone health, and it does this by altering the way that calcium-transporting proteins, amongst others are made. As we discussed in the last module, all tissues in the body break down and rebuild themselves constantly as a means of repairing damage and staying ‘new’, and bone is no exception. Without adequate intakes of calcium and phosphate, bone density will reduce and without vitamin D these two minerals cannot be absorbed, even if intake is theoretically adequate. This is why deficiency in vitamin D causes rickets – a bone deformity which typically manifests as bow leggedness, caused by insufficient sunlight exposure (thus vitamin D) during childhood and adolescence. It is also worth noting that genetic defects and other health conditions may also be responsible.
Aside from that, vitamin D and the gene expressive effects it holds are also involved with immune function, muscle function, heart function, brain development and your respiratory system, while additionally having the ability to reduce cancer risk. Alongside rickets, deficiency is also associated with depression and low mood (especially prevalent in the winter in the Northern Hemisphere).
Research into vitamin D is increasing in recent years, with many further exciting potential effects being shown, including but not limited to improved body composition, improving fall risks in the elderly and increased testosterone levels in men. It’s disease and developmental abnormality risk mediating properties are only just being elucidated, but early evidence is showing that vitamin D supplementation may even be protective against chromosomal abnormalities.
Skin synthesis of vitamin D is able to meet minimum requirements after around 30 minutes of constant exposure to relatively strong sunlight on the arms and face, but this process is hampered by some key factors:
- Outside of the band around the equator stretching from the latitudes 42 degrees North and 42 degrees South you aren’t likely to get sunlight strong enough for most if not all of the year. Note that Lands’ End has a latitude of 50 degrees North and John O’Groats is at 58, so the UK falls outside of that range. Outside of this range, at least in Winter, synthesis can be close to if not zero due to low light levels
- As we age we experience thinning of the skin, which makes this process less effective
- Clothing and sunscreen use both impact the amount of UV rays which come into contact with the skin. Of course, in colder months in countries outside of the abovementioned band, clothing is worn more which results in even less exposure
- Skin pigmentation affects vitamin D synthesis. The darker your skin, the less likely it is that UV light will reach the required dermal layer
Overconsumption of vitamin D is very, very unlikely but it is indeed possible with very large dose supplementation.
Toxicity results in, amongst other things, elevated levels of calcium in the blood which may lead to calcification of soft tissues including kidneys, heart, lungs and blood vessels. For shorter-term hypercalcaemia you would be likely to experience loss of appetite, weight loss, weakness, fatigue, vomiting, constipation and irritability. During pregnancy it can cause unborn infants to have congenital heart problems, elfin faces and cognitive developmental issues.
| Population | RNI | UK RNI | TUL |
|---|---|---|---|
| Adult males (19-50*) | 5ug** | 10ug | 100ug*** |
| Adult females (19-50*) | 5ug | 10ug | 100ug*** |
| Pregnant women | 5ug | 10ug | 100ug*** |
| Lactating women | 5ug | 10ug | 100ug*** |
* From 50+ this intake level doubles to 10ug
** Vitamin D is often stated in IU. 1ug of Vitamin D is equivalent to 40IU, so 10ug is 400IU
*** There is a NOAEL (No Observed adverse effect level) intake of 250ug per day in men, but this lower figure acts as a large safety buffer. In fact, some potentially beneficial doses exceed this lower band, but do not meet the higher one.
Note: For reference, 85g sardines has 6.5ug, half a pint of whole milk has around 4 and an egg has around 1 and a half.
The above levels should be sufficient to maintain proper calcium levels and maintain normal function, but it should be noted that a higher intake of vitamin D may offer more benefits. Vitamin D is one of the vitamins whereby the intake to avoid deficiency may not be as high as the intake level which is ‘optimal’ and various bits of research have concluded intake ranges of between 20-80iu per kg of bodyweight per day can improve certain health markers. We tend to recommend those looking to supplement vitamin D to improve their overall health should look to this range, though we strongly advise never exceeding 10,000iu or 250ug.
Vitamin E
Vitamin E comprises a group of 8 similar homologues (things which are kind of the same but different) grouped into tocopherols and tocotrienols, each of which is broken into A, B, Y and D forms (alpha, beta, gamma, delta). This is important, because it is alpha-tocopherol (A-T) which is the most easily absorbed form, and much like in the case of vitamin A where retinoid levels are recommended, intake recommendations are expressed as an amount of A-T as opposed to vitamin E in general. The only truly practical purpose for knowing this is if you were going to supplement with vitamin E, in which case a cheap version would either comprise another form, or a synthetically formed dl-a-tocopherol which must be consumed in a higher amount than usual (dl-A-T is about 75% as potent as naturally occurring A-T, for example).
A-tocopherol is present in large amounts in sunflower and palm oil, nuts, avocados, egg yolks, whole grains and green leafy vegetables, and as such deficiency is relatively rare.
Upon ingestion vitamin E is transported via chylomicrons to the liver, before being packaged into other lipoproteins and ultimately transferred to cells where it is stored within the lipid bilayer of tissues.
The primary role vitamin E plays within the body is as a lipid-soluble antioxidant. Antioxidants are often a hot topic within mainstream nutrition, with antioxidant supplements and enriched foods becoming more and more popular. In reality they need not be supplemented in unnaturally high amounts (and in fact doing so could be harmful), but that doesn’t mean they aren’t important. In fact, adequate antioxidant intake is hugely beneficial to disease risk minimisation.
What is an antioxidant?
As you know, atoms have a certain amount of electrons on their outer ring which they ‘prefer’ to maintain, as this keeps them stable. Within the body, oxygen is one of the main molecules for metabolism, and in its naturally occurring state oxygen is in the O2 form, meaning that two oxygen atoms are linked together, like so:
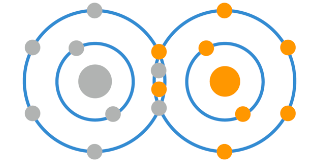
Here you see that the inner ring has 2 electrons, then the outer ring has 6, with each atom sharing 2 of its 6 electrons with the other. It is possible, however, for one of these oxygen atoms to gain an extra electron, like so:
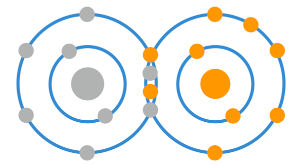
This can happen due to either normal physiological conditions or environmental factors. For example, during the normal processes in cells which result in ATP, oxygen undergoes a process which adds electrons to it in the production of water, but occasionally it doesn’t get all it needs.
To become water, an oxygen molecule needs to get an additional 4 electrons. If it only gets one it becomes a superoxide anion, gets 2 it becomes hydrogen peroxide, and if it gets 3 it becomes a hydroxyl radical. This are commonly referred to as a free radicals, or radical oxygen species (ROS), and in too great a number they can become bad news.
These radicals can be created through unusual conditions including exposure to excessive radiation, stress, inflammation, smoking, alcohol, drugs, chemicals or even a poor diet. These radicals are not all oxygen-based, but the majority of free radicals you are likely to come into contact with are ones which have been produced endogenously through the manufacture of ATP.
These free radicals are highly unstable because of the additional electron(s), and this can cause problems. One of the main mechanisms for this occurring is that they can ‘steal’ an electron from one of the lipids on the lipid bilayer of the cell membrane. Free radicals can also oxidise proteins within a cell, and both of these processes can obviously harm the cell.
Crucially however, these reactive oxygen species can oxidise DNA, causing the kind of mutations that could lead to cancerous growth.
Furthermore, as you know, the oxidation of PUFA found in VLDL and LDL lipoproteins is one of the primary issues which can lead to atherosclerotic plaque.
The first defence against free radicals (oxidants) are antioxidants, which can effectively ‘donate’ an electron to them and render them inert, and therefore neutralise the issue. Antioxidants, therefore, are extremely important (even if, as we said, taking supra-physiological doses of them as supplements probably won’t be of huge benefit, and as you’ll see, you can get all you need from eating normal food, rather than spending your life savings on rare and endangered berries from the Guatemalan jungle).
Once incorporated onto the lipid bilayer of cells, vitamin E can neutralise reactive oxygen species effectively, converting itself into forms which are then collected and excreted in bile.
Levels of Vitamin E are relatively well regulated at high intakes, because to be transported to peripheral tissues in lipoproteins the molecules must be transported to the relevant areas by specialised binding proteins, and this process is self-adjusting according to intake. With that said, deficiency and toxicity are definitely possible.
Vitamin E deficiency can result in muscle and neurological problems, as well as a greater risk of diseases associated with oxidative stress including cancer, neurodegenerative diseases and atherosclerosis. As mentioned above, it is unlikely that someone would become deficient in the Western world, with deficiency generally only appearing in humans during times of famine or in the presence of conditions which impair absorption. With that said, it’s worthy of note that those on very low-fat diets, or very low food intakes in general for the purposes of fat loss may be at some small level of risk.
There is very little data which can be collated to provide vitamin E recommendations, because incidence of deficiency is so rare. In fact, the means of determining intake which is most often utilised is by recommending an amount relative to the average population intake of PUFA (seeing as prevention of PUFA oxidation is probably the primary benefit of vitamin E), with the ration being 0.4mg of a-tocopherol per gram of PUFA.
UK guidelines of 2550 and 1940kcal per day for men and women respectively, each with an intake of PUFA at 6% of intake (so 17 and 13g each) have therefore led to a recommendation of 7 and 5mg of vitamin E for men and women. High intakes of Polyunsaturated Fatty Acids (PUFA) may justify a higher intake, and doses often 100-200mg of the synthetic all-rac-a-tocopherol are common, with no adverse effects. In fact, only doses of over 1000mg per day have ever shown toxicity (which presents as pro-oxidative action). Due to this ambiguity, no WHO RNI is established, and we will not be providing a table like the other vitamins.
In short, 7 and 5 mg are the minimum recommendations for men and women respectively, though a higher intake based upon a higher calorie intake or higher PUFA intake is wise, with around a ratio of 0.4mg of a-tocopherol per gram of PUFA being a reasonable starting place. Overdosing is unlikely without high-dose supplementation, but intakes of over 1000mg have been shown to cause harm.
Note: For reference 25g of almonds has around 7.3mg of Vitamin E, one whole avocado has around 2.7 and a large handful of raw spinach has somewhere around 7mg.
Vitamin K
Vitamin K is the final fat-soluble vitamin, the primary role of which surrounds blood clotting (in fact it’s vitamin ‘K’ because the German word for coagulation starts with a K). As per all of the other fat-soluble vitamins so far, vitamin K refers to a number of different active molecules which have similar but subtly different structures. The group includes phylloquinone, phytonadione and others, but very little information exists to elucidate the actual metabolic differences between the different forms upon ingestion.
After ingestion, it takes the usual route which lipids take via lymphatic fluid and then blood to the liver, where it helps to manufacture a number of coagulation products including ‘factors II, VII, IX and X’ and carboxyglutamate (Gla) which arrest and prevent bleeding by forming blood clots and proteins C and S which do the opposite, by inhibiting blood clotting. Despite this seemingly juxtaposed set of functions, deficiency in vitamin K manifests primarily as a tendency towards excessive bleeding. Vitamin K also seems to be important for certain proteins involved with bone mineralisation, but the actual mechanisms here aren’t very well documented.
After the vitamin K dependent procoagulants (proteins which promote coagulation) are synthesised in the liver, they are secreted in their inactive forms into the blood. In the presence of Gla and calcium these proteins are able to bind to platelets in the blood as well as cells on the inner lining of blood vessels, then when coagulation is initiated these start to form a clot. Protein C effectively blocks this from happening too early, and protein S helps make C more able to perform its job.
Vitamin K deficiency, as mentioned, can lead to excessive bleeding, with this presenting the greatest problem during the first 6 months of infancy. This deficiency is known as haemorrhagic disease, or more recently and more specifically vitamin K deficiency bleeding (VKDB). Maternal drugs which impact on absorption are the primary causes of vitamin K deficiency in infants though other underlying illnesses such as cystic fibrosis may also play a role. Primary sites for bleeding in infants include the intra-abdominal cavity, inside the cranium and within the GI tract, and all can be fatal.
Because of this, it is now common practice for infants to be given vitamin K supplementation if healthcare professionals suspect there is a risk due to low cumulative milk consumption (either breast or formula), very early births and other complications during labour. It has even been suggested that getting enough vitamin K via breastmilk is either very difficult or impossible, and as such an oral or intramuscular supplement is often prescribed.
In adults, vitamin K deficiency which results in bleeding is almost unknown outside of situations caused by underlying pathology, though a low intake can manifest as excessive bleeding when cut, due to a large amount of ‘unfinished’ procoagulant proteins in the blood which are unable to bind to calcium properly.
Excretion of vitamin K happens readily, with large amounts of that which is consumed being removed either in the urine or in faeces via bile, and as such oral dosing of the natural form doesn’t seem to have an upper limit which is toxic. With that said, excessive intakes of synthetic forms given as supplements to infants have been known to lead to liver damage, which suggests an excessive intake does indeed exist, though this is as yet undetermined. What is known is that doses often 20mg or greater do not seem to cause harm, despite being an order of magnitude above the RNI, and it is unlikely that any harm would come at intakes of 1mg or less.
The best dietary sources of vitamin K are green leafy vegetables, olive and rapeseed oils, fermented foods including cheese and animal liver. Additionally, significant amounts of vitamin K are synthesised in the colon by various microbes which live there, including bacteroides, enterobacter and veillonella, all of which are ‘fed’ by a diet rich in vegetable matter.
| Population | RNI | UK RI |
|---|---|---|
| Infants (0-6 months) | 5ug* | N/A |
| Adults | 1ug per kg of bodyweight | 1ug per kg of bodyweight |
* As noted above, this may be difficult to get from breastmilk alone, as infants exclusively given vitamin K from breastmilk tend to get on average 7-13% of their needs daily. This seems to be independent of maternal vitamin K intake, and as such, supplementation via oral or intramuscular administration is often given.Note: For reference, an 80g portion of raw kale has 444ug of vitamin K, spring onions have over 100ug and brussells sprouts have around 100ug.