Protein
The first macronutrient that we will discuss here is protein. ‘Protein’ is named from the ancient Greek word meaning ‘the most important one’, and we tend to agree with that.
Protein is contained in most foods in some amount or other, but the kind of foods which would ideally be emphasised in a diet are those containing ‘high quality protein’ which denotes foods such as meat, fish, poultry, dairy, eggs, offal, tofu, some meat substitutes and some protein powders of both animal and plant varieties. Where possible, animal products should be used (including dairy and eggs) as your main protein sources, preferably in forms which are ‘lean’ (which simply denotes a lower fat content) but plant sources like tofu and powders can be used also.
To be clear, this is of course not to say that vegetarian or vegan diets cannot provide adequate protein intake. This simply takes a little more planning.
While protein is the nutritional term, it is far more accurate to think in terms of ‘proteins’. Animal bodies make millions of proteins every day in order to serve a huge range of functions. The process of making proteins occurs within most cells of your body and is one of the main things which keeps you alive day-to-day.
To give examples, collagen and elastin are 2 proteins which make up your skin, actin and myosin are 2 contractile proteins that allow your skeletal muscle to make you move, and we’ve already discussed enzymes, which account for the vast majority of proteins synthesised on any given day (to synthesise means to make out of smaller parts). In order to show you just how important it is to consume sufficient dietary protein, we’ll talk about what proteins are, how they are made and the way which the food that you eat plays into the whole cycle.
Proteins are made of smaller units called amino acids which are arranged in a linear chain, like a string of beads. Every time a cell makes a protein, what it is doing is linking together a bunch of amino acids in a particular way. Amino acids, structurally, look like this:
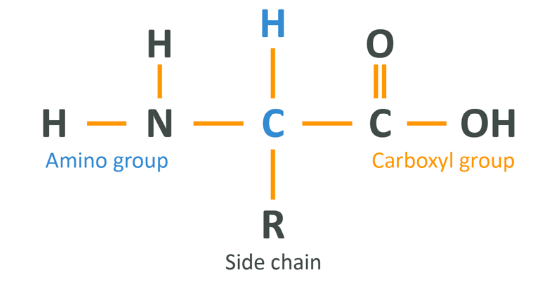
This can be viewed from left to right as such:
- The N-H-H (NH2) is referred to as the Amino Group or N Terminal. It is made up of 2 hydrogens and 1 nitrogen
- The central molecule is carbon, and it attaches to a hydrogen and 1 variable side chain
- The right-hand side is the carboxyl group or C Terminal, which is made up of a carbon attached to 2 oxygens. 1 oxygen is bound in a ‘double bond’ denoted by 2 lines, which means that the 2 atoms share 4 electrons rather than just 2. The other oxygen is attached via a single bond and also attached to a hydrogen which, as we’ll mention in a minute, sometimes separates from the structure
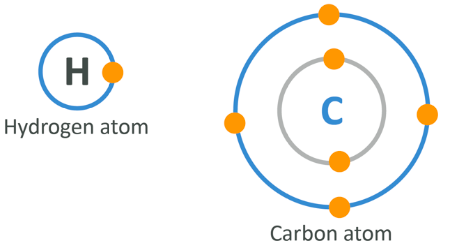
A quick chemistry reminder
Atoms are made up of a nucleus which is itself made up of protons and neutrons, surrounded by electrons which are located in the ‘rings’ of orbit. The outer ring of electrons is what determines how reactive an atom is. To join 2 atoms together, one of two things can happen:
- Either a metal atom and 1 or more non-metal atoms meet, in which case the metal ‘loses’ electrons and becomes positively charged, magnetically, and the non-metal(s) ‘gain(s)’ electrons to become negatively charged and they all become attracted magnetically – a process called ionic bonding, or
- As happens here, 2 atoms ‘share’ a pair of electrons, 1 from each original atom (single bond) or 4 electrons, 2 from each original atom (double bond) from their outer ring. This is called a covalent bond
One thing you need to know for the rest of this module is that carbon has 4 electrons on its outer ring meaning it can make up to 4 bonds, and hydrogen has only 1 outer electron so it can only bond to one thing at a time. Both are non-metals, and as such can only bond through the second process.
When 2 amino acids are linked together, the hydrogen and oxygen from the carboxyl group of 1 amino acid combine with 1 hydrogen from the N Terminal of the next amino acid and are released (as H20 – water – oxygen with 2 hydrogens), leaving behind 2 amino acids linked in what is known as a dipeptide, joined via a peptide bond. As such, a protein chain could be considered to start at the N Terminal of the first amino acid, and then continue until reaching the final C Terminal which is also ‘free’. When a number of dipeptides are linked in a chain the resultant structure is known as a polypeptide.
Diagrammatically, the linking of 2 amino acids looks like this:
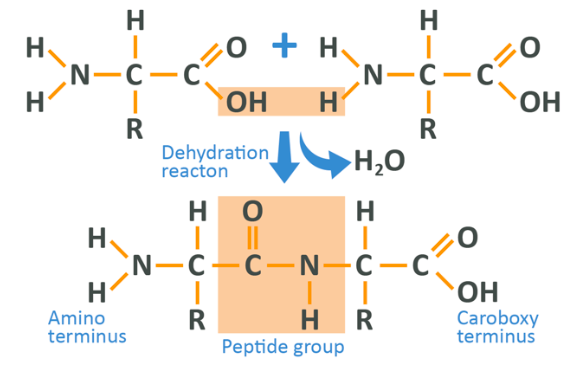
See above that the OH of the left-hand amino acid and the H of the right-hand one have combined to make water (which is therefore ‘lost’). This is the reason this is known as a dehydration reaction, and the remaining molecules have joined together to form the start of a chain. The main thing to remember from this section is that amino acids have a specific common structure, but their side-chains vary. Then when they are linked together they lose a molecule of water and start to form a chain.
Each amino acid, as we’ve discussed, has a side chain – it is this side chain which determines which amino acid it is, and it is this side chain which gives it it’s function. There are 20 different amino acids relevant to humans, each having a unique side chain. They are as follows:
| Essential amino acids: | Non-essential amino acids: |
|---|---|
| Histidine | Glutamine |
| Iso | Alanine |
| Leucine | Arginine |
| Lysine | Aspartate |
| Methionine | Cysteine |
| Phenylalanine | Glutamate |
| Threonine | Glycine |
| Tryptophan | Proline |
| Valine | Serine |
| Tyrosine | |
| Asparagine |
Your cells use these amino acids to make proteins in perhaps one of the most fascinating aspects of biology – gene expression.
DNA is formed out of 2 long strings which form a double helix, which then bundle together into chromosomes. Along each string are single units which follow a particular order – this is your genetic code, and various sections of that code can be thought of as being separated into specific genes. To conceptualise this, think of DNA being a library filled with genes which are books, written in words which are single units. If you want to make something you could go into a library, pick the requisite manual and read the words in order to know what to do. Your cells do the same.
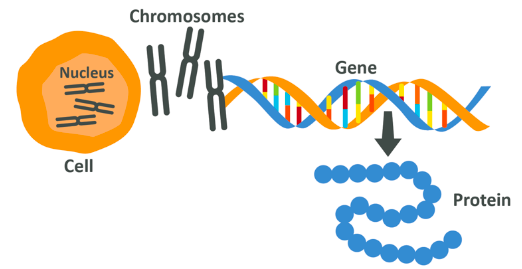
To make a protein, a gene is ‘expressed’ meaning that it’s individual units are read and translated by cell substructures which then start to link together a precise set of amino acids in a specific order. Going along the gene (which is a DNA chain section), each 3 units of DNA information correlate to a specific amino acid. Because of this a gene can be considered to be a set of instructions for making a protein.
Once the cell has read the code and linked together the string of amino acids which it’s been ‘told to’ use, the string will begin to fold in on itself in a specific way depending on the amino acids which it contains. Each amino acid, as you know, has a certain side chain and that side chain, because it’s made up of atoms, will have a specific magnetic field and set of places where binding can occur. All of this means that there is only one shape which that chain can stably adopt.
This means that the folding of the string is precisely predictable from the constituent amino acids, which are in turn predictable from a given section of DNA (a gene). Every time that particular chain of amino acids is formed in that particular order from that particular section of DNA, the final ‘string’ folds in on itself in exactly the same way.
The order of the amino acids in the chain therefore determines the final shape of the protein and it is this 3D shape which ultimately gives a protein it’s function. In fact, many ailments can be chalked up to this process happening ineffectively, resulting in proteins being formed which are misshapen and do not ‘work’ as they should. The diagram below illustrates how a protein forms.
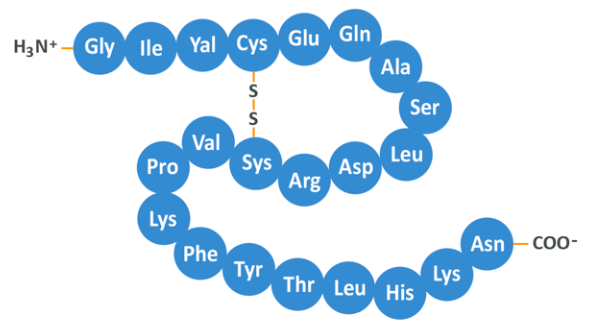
So, DNA codes for amino acids, which link together and then fold up into proteins. The shape of those proteins, along with the chemical properties their surface amino acids have, then allow them to perform a given job, be that to speed up cellular reactions, to help a muscle contract or to make your hair brown. The above is a little complex, but this is what protein ‘is’. When we eat a rump steak, what we are doing is consuming the resultant matter from countless millions of proteins being formed according to the cells present in the butt of a cow. We can then use the amino acids formed therein to perform cellular processes of our own.
Forgetting about nutrition for a second
At all times, as we discussed in the last module very briefly, certain proteins within your body are being broken down as they become damaged or obsolete, and are then being replaced by newer proteins. As you can now probably deduce, the proteins are broken up into their amino acids, most of those amino acids are then recycled (some are broken down further and the individual atoms are used elsewhere), and they can be recombined into either the same or different end-products. Say, for example, your body breaks up a slightly damaged collagen protein which was a part of a tendon. The amino acids therein could be used to synthesise another collagen to use elsewhere, they could be used to make a completely different protein that might be needed at the time or they could be sent to your liver to be broken down and made into something else entirely. Your body really is incredible.
If your aim is to build muscle mass, what that means is that the cells in your muscles need to have the amino acids on-hand that they need to synthesise new muscle proteins.
Now, look back at the list of amino acids and notice that they are grouped into two categories – essential amino acids and non-essential amino acids. Don’t let the names lead you to think that non-essential is not important. Essential, in terms of nutrition, simply means that it must be consumed as it cannot be synthesised. As you have seen, an amino acid is just some nitrogen, carbon, hydrogen and oxygen atoms smashed together in a particular way with a variable side chain, and as such we are able to break down amino acids, sugars and other things to ‘build’ amino acids that we need. We unfortunately lack the necessary mechanisms to be able to build certain amino acids though, and as such, we need to get them from food.
When we talk about foods with ‘complete’ protein profiles or proteins that are high ‘quality’ we are referring to them containing the 9 essential amino acids in ratios which make them useful for building proteins in our body. A complete protein source is one which allows our cells to perform the above routine when required, because they have all of the necessary ‘building blocks’… and that is why dietary protein is important. Without it, you would not be able to synthesise the proteins which make up your skin, organs, muscles, enzymes or blood, nor the proteins which are needed so that your cells can communicate with each other. Protein deficiency would lead to an effective shutting down of most of your metabolic processes and ultimately death. This is illustrated when scientists feed animals an artificial diet containing only collagen protein which is very low in isoleucine, threonine and methionine while being completely void of tryptophan, which are essential amino acids.
When you eat a food containing protein, for the sake of this example we’ll say a steak, first you chew it to break it down into smaller chunks with a greater surface area, and then you swallow it into the oesophagus and eventually the stomach. When the steak reaches the stomach, it comes into contact with the inactive enzyme precursor pepsinogen which is rapidly activated to become the active enzyme pepsin. Pepsin breaks the bonds in between sections of amino acids, leaving slightly shorter chains or polypeptides which then pass through the pyloric sphincter and into the first part of the small intestine known as the duodenum. The duodenum is the main site for nutrient absorption and digestion.
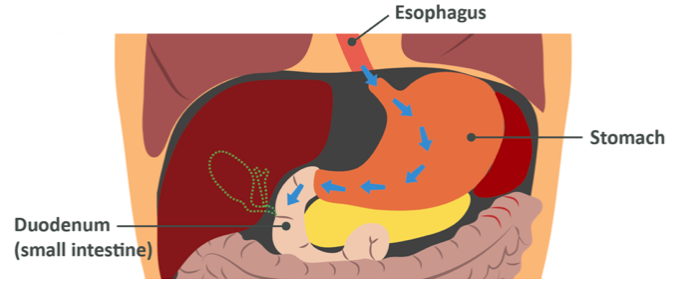
Once the polypeptides are in the duodenum, the pancreas introduces 3 more enzymes called trypsin, chymotrypsin and carboxypeptidase (car-boxy-pep-tie-daze) along with bicarbonate ions which are alkali to neutralise the stomach acid – if this did not happen the proteases here would be damaged. These break down the peptide bonds further, leaving us with very small polypeptides only a few amino acids in length.
The cells on the walls of the small intestine are called enterocytes and they look like the below. What you see here is a surface which has small protrusions on it known as villi, which act to increase surface area. This increased surface area allows for greater rates of absorption from the small intestine. On these small protrusions are even smaller ‘hairs’ called microvilli, and amongst these you will find special enzymes called brush-border enzymes.
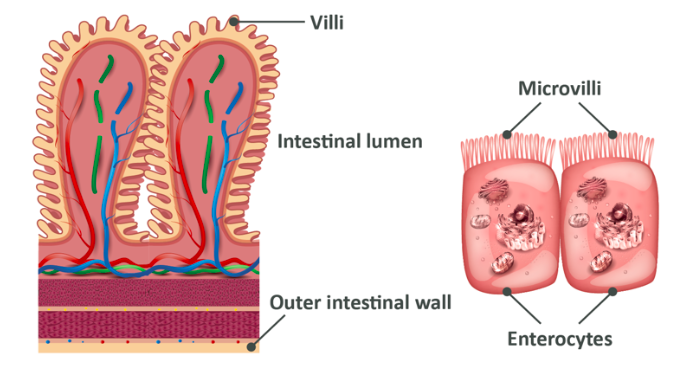
These brush border enzymes are responsible for the final stage of breaking proteins down into amino acids, di and tri peptides which can all be absorbed into the cells. Once inside the cells, the di and tri peptides are broken down into single amino acids and finally they pass through the other side and into the blood.
Once the amino acids are in the blood, they are taken first to the liver to either be used to make liver proteins, broken down into glucose or put back in the blood to be distributed around the body for cellular processes. The amount of protein which is turned into glucose via a complex series of reactions called gluconeogenesis (gluco-neo-genesis) varies depending on how many grams of protein you just ate as well as how many grams of carbohydrates and how many total calories you eat. All you need to remember here is that excess protein eaten above levels which you need to maintain bodily tissues and build muscle is converted to glucose and used as glucose, meaning that you can’t really eat too much protein, and that excess protein eaten above need is not simply wasted but converted to glucose to use to make energy.
Similarly, in between meals when blood glucose starts to drop, your body is able to release amino acids from proteins and re-absorb them into the blood via the hormone glucagon, which we’ll return to. These amino acids are then absorbed by the cells of the liver and converted into glucose, which can be used to fuel various cells around the body.
As a summary of protein in the body, consider that most structures within your body are made up of proteins, which are themselves made up of strings of amino acids. We are able to make some of these amino acids from other things, but a lot of them we need to get from the things we consume. When we consume them, proteins are broken down in to their constituent amino acids and these are then distributed via the blood to cells where they are required for protein synthesis. Without getting adequate dietary protein, our body must get the amino acids it needs from those which are already stored, which as you can probably deduce means increasing protein breakdown from immediately non-essential things (such as muscle mass beyond the bare minimum) and using this to facilitate protein synthesis in more vital places like your organs and blood. If you eat more protein than you need, it’s converted to glucose and used in the same way as glucose is always used.
Protein in the diet
With the slightly more complex stuff out of the way, let’s talk about the role which protein plays within your diet, and the reasons that it should be included (barring the above-mentioned idea that deficiency will kill you).
Perhaps the most commonly discussed role for protein is its ability to increase muscle protein synthesis, though it may not always be spoken about using that terminology. Protein turnover which we have already outlined happens in every tissue, but the tissue we will focus on for the rest of this module is specifically skeletal muscle, because it is chiefly muscle protein synthesis which you should really concern yourself with for practical purposes. The amount of protein required on a daily basis to cover your needs for organ tissue maintenance and enzyme production is comparatively low, and provided you are eating sufficient calories and a relatively balanced diet you aren’t likely to become deficient.
The main reason we need to talk about protein is because of its impact on skeletal muscle tissue.
Muscle protein synthesis is the process by which amino acids are used to build or repair skeletal muscle specifically. As already discussed, a certain amount of protein is broken down within muscle tissue during a given day, and therefore at various points you will be in fact losing muscle. This is not something to be concerned about, however.
Much like it is the case that, calorie balance works over an extended period of time (meaning that you could overeat some days and under-eat others and still lose fat if the net result is a negative), muscle protein balance is cumulative and best thought of over the course of a day (it’s not advisable to consider your protein needs over the course of a week, as muscle protein synthesis can only work ‘so quickly’, and if you severely limit it for 4-5 days, 2 days of high protein intake won’t compensate for it).
Over 24-hours certain things that you do will increase the rate at which protein is broken down – you could be stressed, you could fast for extended periods, you could exercise – and from the beginning of that stressful process to the end you could end up with less skeletal muscle protein. But all is not lost!
Ingesting protein initiates muscle protein synthesis. Assuming your overall protein intake is adequate and you are not in an extreme calorie deficit (this can also be skewed if you have certain health issues or are extremely lean), all of the lost protein is then replaced, and you carry on your life without suffering muscle wastage.
When you perform resistance training, your muscles become somewhat more receptive to dietary protein, and the ceiling for the rate of muscle protein synthesis is increased. Now muscle proteins that are broken down are not only replaced but super-compensated for, resulting in a greater amount of stored protein, and therefore a larger muscle over time.
It’s not just about having available building blocks, though.
Muscle protein synthesis must be ‘activated’ and the key signaller for this response is the consumption of the amino acid Leucine. In research where subjects consumed the same amount of protein, with one group consuming more leucine and the others less, the former group got a greater muscle protein synthetic response, and when two different proteins are compared but the leucine content is matched, the response is equal. It’s a widely accepted fact that leucine is the amino acid mostly needed to activate muscle protein synthesis, and as such we should wherever possible aim to choose proteins with a reasonable leucine content if we want to maximise muscle gain/retention. This is why animal-based protein sources are preferable – while some are better than others, with whey protein and eggs being richer in leucine than pork. For practical purposes this is much of a muchness, and so long as the bulk of your protein intake is from animal-based sources or well-balanced vegan ones (again, something we’ll discuss in later modules) then you have no need to start searching for the leucine content of your foods – this is not something you’ll find on the label!
This isn’t just about making gains, though. If you are losing fat at the minute then that means your overall energy intake will be less than your ‘maintenance’ level. This reduction in calorie intake has the ability to increase muscle protein breakdown and reduce muscle protein synthesis and as such, the ability of dietary protein, especially leucine rich protein, to increase muscle protein synthesis upon ingestion becomes of greater importance. Making sure that we consume enough protein during a dieting phase allows us to safely lose weight without running the risk of excessive muscle loss (of course, as you might guess, resistance training augments this protective effect, too).
So, eating sufficient protein while in a calorie surplus maximises the amount of muscle you can gain from that given surplus with a given training stimulus, and conversely eating sufficient protein while in a calorie deficit allows for minimised muscle tissue loss (which would lead to losses in strength and a comparatively unfavourable body composition relative to the same leanness with more muscle mass).
That isn’t all protein does, though. Some other key roles for dietary protein are:
- Dietary protein has a higher TEF than other nutrients. Because of this, it means that you could theoretically eat the same amount of total calories with a high and low protein intake, but the net result would be lower for the higher protein diet. This is relevant, but not really ground breaking – seeing as the TEF for protein is around 20% and the TEF for carbohydrates is around 10, it probably wouldn’t make a difference to the point that your body composition would likely change to a noticeable degree
- Dietary protein is the nutrient which, calorie for calorie, reduces hunger the most in between meals. In one study, just 20g of whey protein reduced the amount of food participants consumed in a subsequent meal. It does this by increasing the circulation of appetite reducing hormones including GLP-1, peptide YY and cholecystokinin while reducing grehlin, the hormone which makes you hungry. One study found that simply by giving participants a protein intake equal to 30% of calories they were able to reduce their calorie intake by 411 calories without effort!
- Foods which provide protein also come with a ton of important micronutrients, some of which (like Iron) are especially necessary for some individuals to pay attention to
- Increasing your protein intake seems to reduce cravings for other foods, especially late at night which we all know is the time of day where dietary adherence is the hardest. In one study, cravings were reduced by 60% throughout the day by just increasing protein intake to 25% of calories, which could make a massive difference
- Protein can be used to make glucose if needed
How much should we aim for?
According to the UK Eatwell Guide, the current recommended nutritional intake (RNI) of protein for adults aged 19-64 is 0.75g per kilogram of bodyweight per day, calculated to approximately 55.5g per day for the average man and 45g per day for the average woman. This is the amount of protein on average to ensure you do not end up with a negative nitrogen balance (remember that there’s nitrogen in amino acids? If you are wasting more protein than you are building, you end up with increased nitrogen excretion, which is a surrogate measure for protein balance used because measuring protein balance is comparatively difficult). The RNI is therefore enough protein to maintain health for the vast majority of the population.The RNI, however, is calculated to the average, mostly sedentary individual and of course the amount needed to avoid deficiency is not the same as the amount needed to optimise your diet.
The amount of protein that you personally require is largely dependent on your activity levels, exercise modality and calorie balance. Protein synthesis is energy dependent and an increased energy expenditure also increases protein breakdown as amino acids can theoretically be used as fuel. Finally, contracting muscles with large amounts of force or against resistance causes small amounts of damage which further augments the muscle protein breakdown process. As such, those who exercise regularly require more protein to maintain a level protein balance, and slightly more to actually develop larger muscles. At the same time, a given individual would benefit from slightly more protein, the fewer total calories they consume.
Protein requirements are best expressed as a gram amount per kilogram of bodyweight, but there’s a caveat here. We would highly recommend that if you are quite overweight, you consider your protein intake in terms of your ‘goal weight’ or ideal weight according to BMI. Protein requirements are met when you have provided your body with enough amino acids to do what it needs to do, and that’s dictated by your activity levels, food intake and the amount of muscle/fat free bodyweight you have. Additional weight caused by additional fat mass doesn’t increase protein needs but it would skew grams/bodyweight calculations. If your bodyfat is outside of the healthy range, you need to account for that, and ‘goal weight’ is as good a surrogate measure as any.
The protein amount which seems to be beneficial for the general population according to Laymen et al who did a large review on the metabolic functions of protein is 1-1.2g per kilogram of bodyweight, though an overview on the impact of dietary protein on weight loss and weight loss maintenance by Leidy et al suggests that this could increase to 1.2-1.6g per kilogram for individuals looking to lose fat. This was agreed upon at the Protein Summit 2.0 which was a large conference of over 40 nutrition researchers held in 2007, indicating that the numbers recommended by the SACN (UK nutrition body) may be too low to optimise health, although they are certainly enough to avoid becoming deficient. The evidence on this is pretty clear, in isoenergetic diets (multiple diets with the same calorie amount), higher protein diets reliably cause improved body composition and health results even in those who are not performing regular progressive resistance training.
If you are a highly active individual partaking in sport then we need to look at sport specific research. The protein requirements recommended by the International Society for Sports Nutrition, and therefore the ones we recommend are 1.4-2g protein per day per kilo of bodyweight, the higher figure for individuals taking part in resistance training and the lower figure for endurance athletes. If you are looking to maximise hypertrophy (gain the most muscle possible) then this could potentially go a little higher, to 1.8-2.2g per kg or even higher if the individual chooses, but there would be no additional benefit under most circumstances.
It’s worthy of note that intakes higher than this have been cited as potentially useful for muscle mass retention when fat loss is the aim for someone who is already very lean, but this is beyond this course, and is indeed theoretical. It’s unlikely that anyone but the most advanced of athletes would benefit from anything above 2.2g per kilogram.
it just about total daily amount?
You will recall that one of the higher levels of our nutritional pyramid is meal frequency, timing and distribution and we will now cover some aspects of that.
In order to maximise muscle protein synthesis, it appears that one should consume protein approximately every 3-5 hours, totalling 3-5 protein feedings per day spread out relatively evenly. This is because once muscle protein synthesis is maximally stimulated it seems that it cannot be stimulated again until it has returned to baseline, which takes a certain amount of time. These meals should contain at least 20g of protein to maximise the satiety response, but around 2-3g leucine is needed to maximise the muscle synthetic response in the majority of the literature (this number can go as high as 3.5g in some studies). Of course, going back to the fact that leucine isn’t something you’d ever find on a food label, this would be easier to consider in terms of ‘quality protein’, which should be consumed at around 0.25-0.4g per kilo of bodyweight, per meal. Finally, a dose of protein within 2 hours pre and post exercise seems beneficial for creating adaptations to that exercise.
What we would recommend according to all of the above is the following as a ‘perfect protein distribution’:
- 3 main meals containing 0.4-0.5g protein per kilogram (or 2.5-3.5g leucine), 1 post-workout feeding containing the same, and a small snack somewhere (potentially before bed) containing somewhere around 0.3g per kilo. This would give us an ideal total protein intake of around 2g per kg spread out over 5 feedings which maximise protein synthetic response, the maximal amount of protein induced satiety possible and pre/post-workout protein covered. Now for some context
As we keep mentioning, the key to dietary success is dietary adherence, and one of the big factors for this adherence is a reduction in dietary complexity. Looking at the above, you can see that what is scientifically valid and recommended may not be something which you could actually apply in the real world (at least not for your entire life) because it would require an awful lot of preparation and planning, and that isn’t going to happen if you are on holiday, if you’re facing a stressful time at work or if you are generally really busy. In order to simplify things, we need to consider the impact which having ‘the perfect protein distribution’ would actually have. Would it make much of a difference if you got it perfect in comparison to getting it ‘pretty close’?
Firstly, it’s not entirely clear from the research that the proper distribution which in theory maximises muscle hypertrophy, is the same distribution which minimises muscle loss, so if we are talking about someone who is losing fat it gets a lot easier.
In fact, in studies where participants went almost without any food at all on alternative days (alternative caloric restriction, a form of intermittent fasting) they had no problems maintaining muscle mass. It’s pretty clear that if you are resistance training and eating enough protein overall, muscle loss isn’t really a concern (and even if you aren’t resistance training, this probably isn’t going to be too much of a concern for you as you will more than likely not have a huge amount of muscle mass to begin with – the less muscle you have the easier it is to maintain). Then when looking at muscle gain, there is very little conclusive evidence that 4 meals is significantly better than 3, or that 5 is significantly better than 4 for health, body composition or much of anything over the long-term – in theory there would be a difference, but intuitively it won’t be a huge one.
Because of this, meal frequency shouldn’t really be something you worry too much about if you aren’t aiming to be ‘the best of the best’, though breaking your protein out evenly isn’t too taxing and could likely have at least a modest beneficial impact.
Similarly, it’s a huge mistake to think that per meal protein dosing necessarily needs to meet the ‘roughly 3g leucine’ threshold. It’s not the case that it’s all or nothing, and a meal which contains only 1-2g leucine (contained in a relatively small dose of quality protein) isn’t ‘wasted’, albeit just not optimal. Our position is, therefore, that people who are not high level athletes or highly dedicated recreational exercisers should primarily concentrate on consuming somewhere between 1.2 and 1.8g protein per kilogram of bodyweight throughout the day, ideally in doses of over 20g at a sitting to maximise satiety.
To quantify that, an 85kg male could aim for around 135g protein per day, which could be achieved with 3 meals containing 35g protein and a 30g protein snack.
As for pre and post-workout nutrition, again we need to consider the real-world magnitude of difference. While there does seem to be some benefit of consuming protein before and after training, you need to look at the bigger picture. Is it stressful to you or impacting on your life to worry about a pre/post-workout meal? If so, and you aren’t a high level athlete, don’t. It won’t make a huge difference – though if pre-workout nutrition is skipped, it’s not too much to ask to make sure you have something as soon as is convenient/possible after training. This is somewhere that a protein supplement could be used for the sake of convenience.
A highly dedicated recreational exerciser who wants to come a little closer to optimal should do something similar, but with protein at 1.8-2.2g, and maybe consider placing some emphasis on having something within an hour or so of either side of training.
The best way to introduce a greater protein focus into your diet is to consider it the ‘backbone’ of a meal. When deciding what to eat, first think about your lean protein source, and choose protein based snacks wherever possible because it is these which will have the greatest impact on your satiety.
While nutrition is complex, it’s application doesn’t have to be.
The means that you can adopt to realise these recommendations will be spoken about at length in later modules, so for now please just remember the roles and benefits of protein and the recommended intakes.
The safety of a higher protein intake than the RDA
To conclude, it’s a good idea for us to turn our eyes to the possible health impacts of your protein intake as these can sometimes be a concern. There are two things to talk about here:
- Increasing your protein intake to the ones recommended here has never been shown to negatively impact health in any way. Your kidneys may need to do a little extra work in order to process the additional protein metabolites (waste materials from the breakdown of proteins) but they are more than capable of doing this and it will cause no damage. Consider that having 5 cars on London Bridge is more stress than just one, but the bridge isn’t any closer to collapse because of it. There have been a number of trials into the safety of elevated protein intakes, and intakes of up to 4.4g per kg (that’s a lot) had no negative outcomes
- If you increase your protein intake, you will therefore increase your intake of protein-containing foods. It is possible, in this situation, to make better and worse choices. In general, you should opt for leaner, uncured protein sources – but really to discuss this we need to mention the impact of processed and red meats which we’ll go through in turn. According to the WHO, red meats are a likely carcinogen which means that according to their population research, red meats are associated with a higher risk of cancer, but they can’t pinpoint why. It is our view (and that of many) that this should be taken with a pinch of salt, because of certain flaws in the studies presented
Red meat intake is associated with higher risk of cancer, but it’s also associated with lower exercise levels, lower vegetable intakes, more smoking, more alcohol and a number of other risk factors. It would be very difficult to draw a definitive conclusion, and as there is not currently a mechanistic theory as to why this is, we would suggest that consuming red meat 2-3 times per week will be harmless, and a higher amount (assuming a high intake of vegetables/fibre and general healthy lifestyle) probably would be too. Red meat is an excellent source of iron and B vitamins, and while the potential risks of large amounts of red meat consumption means that there more than likely is too much, the best intake for health is very unlikely to be none at all.
As for processed meat (sausage, bacon, pepperoni, ham, for example), the link is a little stronger. Research shows that your risk of colorectal cancer increases by about 18% for every 50g portion you eat, though this is not as worrying as it first appears. The total lifetime risk is your risk of getting a disease just because you are alive, and any increase in risk is expressed as a percentage of that risk. This means that rather than processed meat increasing your risk to 18% at 50g then 36% at 100g and so forth, it means that your lifetime risk which in the UK is about 7.14% as a male (1 in 14 people) will increase by 18% to just over 8%. This is a small increase which we need to consider alongside the above-mentioned lifestyle factors, but it is foolish to ignore it. As such, in order to attain optimal health, we recommend that you minimise your intake of processed and cured meats to as little as is feasible, while also remembering the context of the small impact this will have (for instance, smoking increases your lifetime risk by around 1900%). In the most basic of terms, a bacon sandwich once or twice per week is a very small risk, and it’s really up to you whether you want to take it in the context of a diet which otherwise reduces that risk, but it’s not really advisable to eat bacon every day.
Moving on, let’s talk about dietary fat.